Abstract
Rationale: Risk factors for asthma among farm women are understudied.
Objectives: We evaluated pesticide and other occupational exposures as risk factors for adult-onset asthma.
Methods: Studying 25,814 farm women in the Agricultural Health Study, we used self-reported history of doctor-diagnosed asthma with or without eczema and/or hay fever to create two case groups: patients with atopic asthma and those with nonatopic asthma. We assessed disease-exposure associations with polytomous logistic regression.
Measurements and Main Results: At enrollment (1993–1997), 702 women (2.7%) reported a doctor's diagnosis of asthma after age 19 years (282 atopic, 420 nonatopic). Growing up on a farm (61% of all farm women) was protective for atopic asthma (odds ratio [OR], 0.55; 95% confidence interval [CI], 0.43–0.70) and, to a lesser extent, for nonatopic asthma (OR, 0.83; 95%CI, 0.68–1.02; P value for difference = 0.008). Pesticide use was almost exclusively associated with atopic asthma. Any use of pesticides on the farm was associated only with atopic asthma (OR, 1.46; 95% CI, 1.14–1.87). This association with pesticides was strongest among women who had grown up on a farm. Women who grew up on farms and did not apply pesticides had the lowest overall risk of atopic asthma (OR, 0.41; 95% CI, 0.27–0.62) compared with women who neither grew up on farms nor applied pesticides. A total of 7 of 16 insecticides, 2 of 11 herbicides, and 1 of 4 fungicides were significantly associated with atopic asthma; only permethrin use on crops was associated with nonatopic asthma.
Conclusions: These findings suggest that pesticides may contribute to atopic asthma, but not nonatopic asthma, among farm women.
Keywords: agricultural workers, allergy, asthma, organophosphates, pesticides
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Growing up on a farm is protective against allergic disease as adults; pesticides are associated with respiratory symptoms in farmers.
What This Study Adds to the Field
Pesticides contribute to allergic asthma among farm women, and early-life farm exposures may modulate risks for allergic asthma associated with current animal contact or lifetime pesticide application.
Farmers and other agricultural workers are at increased risk for respiratory diseases, including asthma, as a result of exposure to grains, animals, dusts, and other agricultural exposures (1). Pesticides may contribute to asthma among farmers (2) and insecticide applicators (3), but data are limited. Analyses from the Agricultural Health Study (AHS) have demonstrated that pesticide use, particularly that of organophosphate insecticides, is positively associated with wheeze among both farmers (4) and commercial pesticide applicators (5). Animal data indicate that organophosphates induce airway hyperreactivity, potentially at doses below those causing acetylcholinesterase inhibition (6, 7).
Farm women provide a unique opportunity to explore both the potential protection against asthma and allergic disease from early farming exposures and the potential adverse consequences of agricultural exposures on adult respiratory outcomes. Although some women are born and raised on farms, others may move onto farms as adults after marrying farmers and thus have farming exposures only as adults. Farm women engage in similar farm tasks as men, although with differing intensity (8–10). Although much work has addressed respiratory risks to farmers, little research has explored the respiratory hazards among farm women. In one study of Finnish agricultural workers, women had higher rates of occupational asthma than men (11).
Among Norwegian adults diagnosed with asthma, the ratio of nonatopic to atopic asthma is greater among farmers than among the general non-farming population. (12). This observation, along with the growing interest in classifying adult-onset asthma phenotypes (13, 14), suggests the importance of evaluating risk factors for atopic and nonatopic asthma separately, particularly among agricultural workers. We used enrollment data from the AHS to evaluate pesticides as risk factors for atopic and nonatopic asthma in a cross-sectional analysis among farm women. Some of the results presented here have been previously reported in abstract form (15).
METHODS
The AHS is a prospective cohort of pesticide applicators and their spouses in Iowa and North Carolina (16). In 1993–1997, 32,347 spouses enrolled by completing either a self-administered questionnaire (81%) or a telephone interview (19%). Respondents provided information on demographics, smoking, farming, lifetime pesticide use, and medical history (questionnaires are available at www.aghealth.org/questionnaires.html/).
All women age 20 years and older, with complete information on smoking, asthma history, age, and body mass index, were included in this analysis; male spouses were excluded. We included all subjects who reported a doctor's diagnosis of asthma after age 19 years, and then further subdivided the cases by atopic status based on a self-reported history of doctor-diagnosed eczema or hay fever. Given our interest in adult asthma, we excluded individuals diagnosed with asthma before age 20 years.
Each woman provided information on personal use of 50 specific pesticides, lifetime total years of pesticide use, and frequency of application. Total lifetime days of pesticide application was calculated as the product of the total years of use and the frequency of use. The pesticides were further classified by functional group (i.e., herbicide, insecticide, fungicide, fumigant), by insecticide chemical class (i.e., carbamates, organochlorines, organophosphates), and by the women's pesticide use patterns (9) (i.e., common use only, 1–2 agricultural chemicals, ⩾3 agricultural chemicals). Women also provided information on current farm activities, working with animals, operating farm equipment, and cleaning with solvents.
We used polytomous logistic regression models to evaluate associations between the farming exposures and asthma. By using polytomous regression, we assessed the associations for atopic and nonatopic asthma separately, and formally tested differences between odds ratios (ORs). The base model consisted of age (four categories: 20–39, 40–49, 50–59, ⩾60 yr), state (Iowa, North Carolina), smoking status (current, past, never), body mass index (three categories), and growing up on a farm (yes or no). We assessed each exposure, individually adjusting for the base-model covariates. We restricted our analysis to those exposures with at least five exposed cases. To evaluate potential confounding by related exposures, we adopted the following two-step procedure. First, we assessed the correlation between those exposure variables that were statistically significant individually. Second, for variables that were moderately correlated (i.e., r > 0.3), we added both exposure variables simultaneously to the base model.
We evaluated dose response for lifetime pesticide use variables. For years of pesticide use and frequency of pesticide application, we used the questionnaire categories and collapsed until the highest category contained at least five exposed cases. For lifetime pesticide days, we created tertiles of the exposed population and used the nonexposed as the referent.
To assess interactions between growing up on a farm and agricultural exposures, we added an interaction term to a logistic model that included the base-model covariates and the agricultural exposure. Because growing up on a farm was so strongly associated with atopic asthma in our data, we limited our analysis of interactions to this subgroup. We noted any interaction with a P value less than 0.2.
All statistical analyses were done using SAS version 9.1 (SAS Institute, Inc., Cary, NC) and AHS dataset release P1REL0310.
RESULTS
Among the 25,814 women who provided complete information on asthma status and all other model covariates, we identified 702 adult-onset asthma cases (2.7%): 282 (40%) as having atopic asthma and 420 (60%) as having nonatopic asthma. Women ranged in age from 20 to 88 years (47 ± 12 [mean ± SD]). Subjects with atopic asthma had a similar age distribution to control subjects, wherease those with nonatopic asthma were slightly older (Table 1). Women with asthma, regardless of atopic status, were heavier than control subjects. The prevalence of smoking among women in the cohort was low, with only 10% reporting current smoking. Subjects with nonatopic asthma were slightly more likely to report ever smoking than control subjects or those with atopy. Women with asthma were more likely than control subjects to report other respiratory diseases, particularly chronic bronchitis. Growing up on a farm was protective against both atopic and nonatopic asthma, with the greater effect for atopic asthma (P value for difference = 0.008). The base model–adjusted ORs were 0.55 (95% confidence interval [CI], 0.43–0.70) for atopic asthma and 0.83 (95% CI, 0.68–1.02) for nonatopic asthma. Growing up on a farm confounded the ORs for the pesticides and was included in all subsequent models.
TABLE 1.
DEMOGRAPHIC, SMOKING, AND FARMING CHARACTERISTICS OF 25,814 FARM WOMEN IN THE AGRICULTURAL HEALTH STUDY, BY ATOPIC ASTHMA STATUS, 1993–1997
| Patients with Asthma
|
|||
|---|---|---|---|
| Control Subjects (n = 25,112)
|
Atopic (n = 282)
|
Nonatopic (n = 420)
|
|
| Characteristic | n (%)* | n (%)* | n (%)* |
| Age, yr | |||
| 20–39 | 7,722 (31) | 77 (27) | 70 (17) |
| 40–49 | 7,090 (28) | 91 (32) | 116 (28) |
| 50–59 | 6,093 (24) | 62 (22) | 127 (30) |
| 60–69 | 3,416 (14) | 39 (14) | 88 (21) |
| ⩾70 | 791 (3) | 13 (5) | 19 (5) |
| Body mass index, kg/m2 | |||
| <23 | 7,703 (31) | 55 (20) | 81 (19) |
| 23–27 | 10,313 (41) | 102 (36) | 148 (35) |
| ⩾28 | 7,096 (28) | 125 (44) | 191 (46) |
| Education | |||
| Less than high school | 1,142 (5) | 8 (3) | 27 (8) |
| High school graduate | 9,077 (40) | 96 (40) | 142 (41) |
| More than high school | 12,323 (55) | 135 (57) | 178 (51) |
| Race | |||
| White | 24,491 (98) | 279 (99) | 404 (96) |
| Nonwhite | 583 (2) | 3 (1) | 16 (4) |
| State | |||
| Iowa | 17,760 (71) | 188 (67) | 287 (68) |
| North Carolina | 7,352 (29) | 94 (33) | 133 (32) |
| Smoking history | |||
| Smoke status | |||
| Never smoked | 18,308 (73) | 199 (71) | 268 (64) |
| Past smoker | 4,270 (17) | 58 (21) | 108 (26) |
| Current smoker | 2,534 (10) | 25 (9) | 44 (11) |
| Years smoked | |||
| Never smoked | 18,308 (73) | 199 (71) | 268 (64) |
| 1–7 | 2,194 (9) | 32 (11) | 45 (11) |
| 8–19 | 2,177 (9) | 18 (6) | 39 (9) |
| ⩾20 | 2,335 (9) | 33 (12) | 66 (16) |
| Medical history | |||
| Chronic bronchitis | 825 (3) | 91 (32) | 106 (25) |
| Emphysema | 47 (0) | 5 (2) | 15 (4) |
| Farmers lung | 35 (0) | 3 (1) | 4 (1) |
| Atopy† | 3,086 (12) | 282 (100) | 0 (0)‡ |
| Eczema | 1,170 (5) | 62 (22) | 0 (0)‡ |
| Hay fever | 2,123 (9) | 245 (87) | 0 (0)‡ |
| Farming characteristics | |||
| Grew up on farm§ | |||
| No | 9,774 (39) | 150 (53) | 172 (41) |
| Yes | 15,338 (61) | 132 (47) | 248 (59) |
| Years worked/lived on farm | |||
| 0–22 | 8,285 (33) | 99 (35) | 121 (29) |
| 23–40 | 8,452 (34) | 103 (37) | 141 (34) |
| ⩾41 | 8,075 (33) | 78 (28) | 156 (37) |
| Days worked in field during last growing season | |||
| None | 12,054 (49) | 138 (50) | 214 (52) |
| <10 | 4,955 (20) | 49 (18) | 84 (20) |
| 10–30 | 4,363 (18) | 50 (18) | 65 (16) |
| 31–100 | 2,808 (11) | 33 (12) | 45 (11) |
| >100 | 667 (3) | 7 (3) | 6 (1) |
| Job off the farm | 22,160 (89) | 258 (92) | 381 (91) |
Percentages based on numbers responding to the question; missing values are not included.
Atopy defined as doctor diagnosis of eczema or hay fever.
By definition, the nonatopic group includes no people with atopy, eczema, or hay fever.
Based on response to the question: “Did you spend more than half your life under age 18 on a farm?”
More than half of farm women reported ever using pesticides (Table 2). Use of any pesticide on the farm was associated with atopic asthma (OR, 1.46; 95% CI, 1.14–1.87), but not nonatopic asthma (OR, 1.00; 95% CI, 0.82–1.22), and the two ORs were significantly different (P = 0.020). Use of herbicides and use of insecticides were associated with atopic, but not nonatopic, asthma. Three classes of insecticides (carbamates, organochlorines, and organophosphates) were each associated with atopic asthma, but not with nonatopic asthma; only for carbamates was the differential association significant (P = 0.014). We saw little evidence for an association with fumigants, potentially owing to the small number of users.
TABLE 2.
ODDS RATIOS FOR PESTICIDE USE AND ASTHMA AMONG 25,814 FARM WOMEN IN THE AGRICULTURAL HEALTH STUDY, 1993–1997
| Patients with Asthma
|
||||||
|---|---|---|---|---|---|---|
| Control Subjects (n = 25,112)
|
Atopic (n = 282)
|
Nonatopic (n = 420)
|
P Value for Difference‡ | |||
| Exposure | n (%)* | n (%)* | OR (95% CI)† | n (%)* | OR (95% CI) | |
| Any pesticide | 14,346 (57) | 181 (64) | 1.46 (1.14–1.87) | 240 (57) | 1.00 (0.82–1.22) | 0.020 |
| Herbicides | 9,394 (38) | 122 (44) | 1.43 (1.12–1.83) | 161 (39) | 1.09 (0.89–1.33) | 0.088 |
| 2,4-d | 3,745 (15) | 52 (19) | 1.53 (1.12–2.10) | 66 (16) | 1.07 (0.82–1.41) | 0.092 |
| Alachlor | 1,058 (4) | 10 (4) | 0.95 (0.50–1.79) | 18 (4) | 0.95 (0.59–1.55) | 0.981 |
| Atrazine | 1,142 (5) | 12 (4) | 1.06 (0.59–1.91) | 18 (5) | 0.88 (0.54–1.42) | 0.627 |
| Butylate | 352 (1) | 5 (2) | 1.45 (0.59–3.55) | 7 (2) | 1.10 (0.51–2.34) | 0.638 |
| Chlorimuron-ethyl | 424 (2) | 4 (2) | 5 (1) | 0.68 (0.28–1.65) | ||
| Cyanazine | 725 (3) | 6 (2) | 0.84 (0.37–1.90) | 14 (4) | 1.09 (0.63–1.88) | 0.596 |
| Dicamba | 1,014 (4) | 11 (4) | 1.11 (0.60–2.05) | 13 (3) | 0.74 (0.42–1.30) | 0.337 |
| Glyphosate | 8,468 (34) | 106 (39) | 1.31 (1.02–1.67) | 148 (36) | 1.13 (0.92–1.39) | 0.376 |
| Imazethapyr | 767 (3) | 3 (1) | 6 (2) | 0.46 (0.20–1.03) | ||
| Metolachlor | 830 (3) | 8 (3) | 0.98 (0.48–2.00) | 9 (2) | 0.64 (0.33–1.25) | 0.393 |
| Metribuzin | 449 (2) | 4 (2) | 6 (2) | 0.71 (0.31–1.61) | ||
| Paraquat | 286 (1) | 6 (2) | 1.90 (0.83–4.34) | 8 (2) | 1.60 (0.79–3.28) | 0.756 |
| Pendimethalin | 606 (2) | 4 (2) | 8 (2) | 0.78 (0.39–1.59) | ||
| Petroleum oil | 894 (4) | 11 (4) | 1.20 (0.65–2.22) | 22 (6) | 1.45 (0.93–2.25) | 0.627 |
| Trifluralin | 1,347 (6) | 15 (6) | 1.14 (0.67–1.94) | 22 (6) | 0.93 (0.60–1.45) | 0.569 |
| Insecticides | 10,052 (40) | 132 (47) | 1.43 (1.12–1.81) | 168 (40) | 0.96 (0.78–1.17) | 0.011 |
| Carbamates | 7,953 (32) | 110 (39) | 1.46 (1.14–1.86) | 136 (33) | 0.98 (0.79–1.21) | 0.014 |
| Carbaryl | 7,776 (31) | 106 (38) | 1.41 (1.10–1.80) | 128 (31) | 0.92 (0.74–1.13) | 0.009 |
| Carbofuran | 463 (2) | 9 (3) | 1.92 (0.98–3.77) | 11 (3) | 1.32 (0.72–2.43) | 0.413 |
| Organophosphates | 6,592 (26) | 91 (33) | 1.45 (1.12–1.87) | 119 (29) | 1.07 (0.86–1.33) | 0.072 |
| Chlorpyrifos | 1,003 (4) | 14 (5) | 1.36 (0.79–2.33) | 16 (4) | 0.96 (0.58–1.59) | 0.355 |
| Coumaphos | 319 (1) | 7 (3) | 2.19 (1.02–4.69) | 8 (2) | 1.43 (0.70–2.91) | 0.417 |
| Dichlorvos | 666 (3) | 9 (3) | 1.35 (0.69–2.66) | 15 (4) | 1.25 (0.73–2.11) | 0.851 |
| Diazinon | 2,542 (10) | 33 (12) | 1.23 (0.85–1.77) | 39 (10) | 0.92 (0.66–1.29) | 0.256 |
| Fonofos | 468 (2) | 8 (3) | 1.80 (0.88–3.68) | 10 (3) | 1.21 (0.64–2.31) | 0.422 |
| Malathion | 5,004 (20) | 76 (28) | 1.60 (1.22–2.10) | 100 (24) | 1.18 (0.94–1.49) | 0.092 |
| Parathion | 240 (1) | 7 (3) | 2.88 (1.34–6.20) | 6 (2) | 1.43 (0.63–3.25) | 0.215 |
| Phorate | 481 (2) | 10 (4) | 2.04 (1.07–3.88) | 9 (2) | 1.01 (0.52–1.98) | 0.136 |
| Terbufos | 732 (3) | 11 (4) | 1.52 (0.82–2.81) | 11 (3) | 0.86 (0.47–1.59) | 0.198 |
| Organochlorines | 1,950 (8) | 31 (11) | 1.57 (1.07–2.31) | 43 (11) | 1.16 (0.83–1.60) | 0.226 |
| Aldrin | 206 (1) | 2 (1) | 6 (2) | 1.39 (0.61–3.17) | ||
| Chlordane | 1,056 (4) | 16 (6) | 1.43 (0.85–2.38) | 25 (6) | 1.25 (0.82–1.89) | 0.688 |
| DDT | 920 (4) | 16 (6) | 1.79 (1.06–3.03) | 26 (7) | 1.38 (0.91–2.08) | 0.437 |
| Heptachlor | 198 (1) | 1 (0) | 5 (1) | 1.22 (0.50–3.00) | ||
| Lindane | 380 (2) | 8 (3) | 1.92 (0.94–3.92) | 7 (2) | 1.00 (0.47–2.14) | 0.218 |
| Pyrethroids | 1,222 (5) | 18 (7) | 1.46 (0.90–2.37) | 25 (6) | 1.34 (0.88–2.02) | 0.787 |
| Permethrin (animals) | 891 (4) | 15 (6) | 1.71 (1.01–2.91) | 15 (4) | 1.09 (0.65–1.85) | 0.235 |
| Permethrin (crops) | 491 (2) | 7 (3) | 1.30 (0.61–2.78) | 17 (4) | 2.19 (1.33–3.61) | 0.254 |
| Fungicides | 1,208 (5) | 18 (7) | 1.41 (0.87–2.30) | 27 (7) | 1.29 (0.86–1.92) | 0.774 |
| Captan | 550 (2) | 8 (3) | 1.33 (0.65–2.70) | 14 (3) | 1.42 (0.83–2.45) | 0.875 |
| Chlorothalonil | 237 (1) | 5 (2) | 1.93 (0.79–4.75) | 5 (1) | 1.20 (0.49–2.94) | 0.457 |
| Maneb | 382 (2) | 8 (3) | 1.93 (0.94–3.98) | 7 (2) | 0.96 (0.45–2.07) | 0.190 |
| Metalaxyl | 366 (2) | 10 (4) | 2.61 (1.35–5.04) | 8 (2) | 1.29 (0.63–2.66) | 0.153 |
| Fumigants§ | 441 (2) | 5 (2) | 1.02 (0.42–2.50) | 12 (3) | 1.57 (0.87–2.82) | 0.428 |
| 80/20 mix | 133 (1) | 1 (0) | 5 (1) | 1.85 (0.75–4.58) | ||
| Methyl bromide | 301 (1) | 3 (1) | 6 (1) | 1.21 (0.53–2.77) | ||
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Percentages based on numbers responding to the question; missing values are not included.
Models adjusted for age, state, smoking status, body mass index, and “grew up on farm;” ORs not reported if fewer than five exposed cases.
P value for the test formally comparing the ORs for atopic and nonatopic asthma.
Fumigant category is based on use of any fumigant; only those pesticides with five or more exposed cases are presented.
Pesticide use was primarily associated with atopic asthma. Although only 1 of 39 analyzed pesticides (permethrin on crops) was significantly associated with nonatopic asthma, 10 of 31 analyzed pesticides were significantly associated with atopic asthma. These 10 were 2 herbicides (2,4-d and glyphosate), 7 insecticides (carbaryl, coumaphos, DDT, malathion, parathion, permethrin on animals, and phorate), and 1 fungicide (metalaxyl). Only the ORs for carbaryl were significantly different between atopic and nonatopic cases (P = 0.009). Individual organophosphate insecticides had the strongest associations with atopic asthma, with the ORs for parathion (OR, 2.88; 95% CI, 1.34–6.20), coumaphos (OR, 2.19; 95% CI, 1.02–4.69), and phorate (OR, 2.04; 95% CI, 1.07–3.88) being the highest observed. The two most commonly used insecticides among the farm women, malathion and carbaryl, were also associated with atopic asthma (ORmalathion, 1.60; 95% CI, 1.22–2.10 and ORcarbaryl, 1.41; 95% CI, 1.10–1.80). The use patterns of malathion and carbaryl are similar, so these variables were correlated. When we included both malathion and carbaryl in the same model, the ORs for atopic asthma were attenuated (ORmalathion, 1.47; 95% CI, 1.08–2.02; ORcarbaryl, 1.19; 95% CI, 0.90–1.59). No other pesticides were highly correlated.
Dose–response models for pesticide use and asthma among farm women were consistent with increased atopic asthma associated with ever using pesticides but provided little evidence that risk continued to increase with ever-greater duration or frequency of use (Table 3). Women who used three or more agricultural chemicals in their lifetime were more likely to have atopic asthma (OR, 1.63; 95% CI, 1.09–2.45) than women who had never used pesticides. We detected no associations between the pesticide duration parameters and nonatopic asthma.
TABLE 3.
DOSE–RESPONSE ESTIMATES FOR LIFETIME PESTICIDE USE AND ASTHMA AMONG FARM WOMEN IN THE AGRICULTURAL HEALTH STUDY, 1993–1997
| Patients with Asthma
|
|||||
|---|---|---|---|---|---|
| Control Subjects (n = 25,112)
|
Atopic (n = 282)
|
Nonatopic (n = 420)
|
|||
| Exposure | n (%)* | n (%)* | OR (95% CI)† | n (%)* | OR (95% CI)† |
| Years on the farm | |||||
| 0–22 | 8,285 (33) | 99 (35) | 1.00 | 121 (29) | 1.00 |
| 23–40 | 8,452 (34) | 103 (37) | 1.32 (0.96–1.83) | 141 (34) | 1.00 (0.76–1.31) |
| 41–90 | 8,075 (33) | 78 (28) | 1.17 (0.74–1.84) | 156 (37) | 0.85 (0.60–1.19) |
| Farm women hierarchical pesticide classification (9) | |||||
| None | 10,766 (45) | 101 (37) | 1.00 | 180 (45) | 1.00 |
| Low toxicity only | 7,400 (31) | 89 (33) | 1.38 (1.04–1.85) | 111 (28) | 0.93 (0.73–1.19) |
| 1–2 Agricultural chemicals | 3,410 (14) | 47 (17) | 1.61 (1.13–2.29) | 67 (17) | 1.14 (0.86–1.52) |
| ⩾3 Agricultural chemicals | 2,436 (10) | 33 (12) | 1.63 (1.09–2.45) | 44 (11) | 1.00 (0.72–1.41) |
| Total years of pesticide use | |||||
| 0 | 10,766 (50) | 101 (42) | 1.00 | 180 (49) | 1.00 |
| 1 | 1,035 (5) | 14 (6) | 1.55 (0.88–2.74) | 15 (4) | 0.96 (0.56–1.64) |
| 2–5 | 2,831 (13) | 30 (13) | 1.26 (0.83–1.91) | 44 (12) | 1.04 (0.74–1.45) |
| 6–10 | 2,051 (10) | 33 (14) | 2.02 (1.35–3.02) | 41 (11) | 1.29 (0.91–1.82) |
| 11–20 | 2,462 (12) | 32 (13) | 1.61 (1.07–2.41) | 45 (12) | 1.13 (0.81–1.58) |
| 21–30 | 1,295 (6) | 19 (8) | 1.79 (1.08–2.97) | 19 (5) | 0.74 (0.46–1.21) |
| ⩾31 | 946 (4) | 11 (5) | 1.34 (0.70–2.56) | 27 (7) | 1.24 (0.81–1.89) |
| Frequency of application, d | |||||
| 0 | 10,766 (50) | 101 (42) | 1.00 | 180 (48) | 1.00 |
| 1–4 | 5,234 (25) | 68 (28) | 1.56 (1.14–2.14) | 91 (24) | 1.07 (0.83–1.39) |
| 5–9 | 2,433 (11) | 33 (14) | 1.62 (1.09–2.42) | 51 (14) | 1.23 (0.89–1.68) |
| 10–19 | 1,856 (9) | 22 (9) | 1.43 (0.90–2.28) | 33 (9) | 1.04 (0.71–1.51) |
| ⩾20 | 1,104 (5) | 16 (7) | 1.78 (1.04–3.04) | 18 (5) | 0.96 (0.59–1.58) |
| Lifetime days of pesticide application | |||||
| 0 | 10,766 (51) | 101 (42) | 1.00 | 180 (49) | 1.00 |
| 1–20 | 3,699 (17) | 50 (21) | 1.62 (1.15–2.30) | 64 (17) | 1.14 (0.85–1.52) |
| 21–105 | 3,190 (15) | 38 (16) | 1.43 (0.98–2.09) | 56 (15) | 1.05 (0.77–1.43) |
| ⩾106 | 3,673 (17) | 50 (21) | 1.65 (1.17–2.34) | 71 (19) | 1.07 (0.81–1.42) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Percentages based on numbers responding to the question; missing values are not included.
ORs adjusted for age, state, smoking status, body mass index, and “grew up on farm.”
We also had information regarding current farm activities, such as animal contact, using farm equipment, and performing farm tasks (Table 4). We saw little evidence of association of these current activities with asthma. Driving trucks on farms was associated with nonatopic asthma (OR, 1.30; 95% CI, 1.07–1.59) but not atopic asthma (OR, 0.93; 95% CI, 0.72–1.19; P value for difference = 0.037). Planting was associated with nonatopic asthma (OR, 1.26; 95% CI, 1.00–1.57), whereas applying chemical fertilizer was associated with atopic asthma (OR, 1.45; 95% CI, 1.03–2.04). These current farming activities, including animal exposures, did not confound the associations with pesticides. For women who ever held jobs off the farm (89%), information regarding off-the-farm occupational exposure to both organic (cotton, wood, and grain) and inorganic dusts (asbestos, mineral, and silica) were available (data not shown). Of these exposures, only wood dust was significantly associated with atopic asthma (OR, 1.82; 95% CI, 1.02–3.25), and only silica dust with nonatopic asthma (OR, 2.25; 95% CI, 1.24–4.08). These exposures did not confound the observed pesticide associations.
TABLE 4.
ODDS RATIOS FOR CURRENT FARM EXPOSURES AND ASTHMA AMONG FARM WOMEN IN THE AGRICULTURAL HEALTH STUDY, 1993–1997
| Patients with Asthma
|
|||||
|---|---|---|---|---|---|
| Control Subjects (n = 25,112)
|
Atopic (n = 282)
|
Nonatopic (n = 420)
|
|||
| Exposure | n (%)* | n (%)* | OR (95% CI)† | n (%)* | OR (95% CI)† |
| Work with animals | |||||
| Any animal | 13,316 (54) | 143 (52) | 1.03 (0.80–1.33) | 189 (46) | 0.85 (0.69–1.04) |
| Dairy | 2,002 (8) | 14 (5) | 0.64 (0.37–1.10) | 27 (7) | 0.90 (0.61–1.34) |
| Beef | 7,952 (33) | 90 (33) | 1.08 (0.84–1.41) | 109 (27) | 0.84 (0.67–1.05) |
| Hogs | 7,292 (30) | 68 (25) | 0.84 (0.63–1.12) | 96 (24) | 0.84 (0.66–1.08) |
| Poultry | 2,778 (12) | 33 (12) | 1.07 (0.74–1.55) | 40 (10) | 0.91 (0.66–1.27) |
| Sheep | 1,510 (6) | 22 (8) | 1.40 (0.90–2.19) | 18 (4) | 0.78 (0.48–1.25) |
| Veterinary procedures | 3,090 (13) | 35 (13) | 1.10 (0.76–1.59) | 35 (9) | 0.76 (0.53–1.08) |
| Farm equipment | |||||
| Combine | 2,787 (11) | 32 (12) | 1.17 (0.80–1.70) | 47 (11) | 1.10 (0.81–1.51) |
| Diesel tractor | 8,153 (34) | 87 (32) | 1.02 (0.79–1.33) | 115 (29) | 0.84 (0.67–1.06) |
| Gas tractor | 6,378 (26) | 65 (24) | 0.94 (0.71–1.26) | 95 (24) | 0.86 (0.68–1.09) |
| Trucks | 9,136 (38) | 97 (36) | 0.93 (0.72–1.19) | 171 (43) | 1.30 (1.07–1.59) |
| Farm tasks | |||||
| Apply chemical fertilizer | 2,696 (11) | 41 (15) | 1.45 (1.03–2.04) | 52 (13) | 1.13 (0.83–1.52) |
| Apply manure | 2,950 (12) | 36 (13) | 1.15 (0.80–1.64) | 55 (13) | 1.12 (0.84–1.50) |
| Grind feed | 1,381 (6) | 12 (4) | 0.82 (0.46–1.47) | 15 (4) | 0.69 (0.41–1.17) |
| Grind metal | 301 (1) | 2 (1) | 5 (1) | 0.97 (0.40–2.39) | |
| Hand pick | 6,267 (25) | 77 (28) | 1.11 (0.84–1.46) | 123 (30) | 1.21 (0.97–1.51) |
| Plant | 5,852 (24) | 63 (23) | 0.94 (0.70–1.26) | 119 (29) | 1.26 (1.00–1.57) |
| Repair engines | 333 (1) | 5 (2) | 1.43 (0.58–3.50) | 7 (2) | 1.23 (0.58–2.63) |
| Till soil | 6,072 (25) | 59 (21) | 0.92 (0.68–1.23) | 111 (27) | 1.19 (0.95–1.49) |
| Solvent use | |||||
| Gas to clean | 3,714 (16) | 44 (16) | 1.17 (0.84–1.64) | 57 (15) | 0.95 (0.71–1.27) |
| Other solvents to clean | 4,896 (20) | 55 (20) | 1.07 (0.79–1.44) | 77 (19) | 0.95 (0.74–1.23) |
| Paint | 7,928 (33) | 89 (33) | 1.07 (0.82–1.38) | 123 (31) | 0.96 (0.77–1.20) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Percentages based on numbers responding to the question; missing values are not included.
ORs adjusted for age, state, smoking status, body mass index, and “grew up on the farm.”
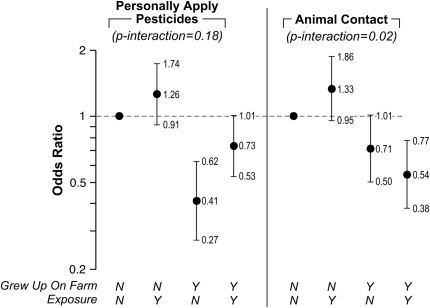
With atopic asthma, we observed statistical interactions between growing up on a farm and both applying pesticides (P = 0.18) and any animal exposure (P = 0.02) (Figure 1). Growing up on a farm was protective against atopic asthma among women who applied pesticides (OR, 0.73; 95% CI, 0.53–1.01); we saw the lowest risk among women who grew up on farms but did not apply pesticides (OR, 0.41; 95% CI, 0.27–0.62). Among the pesticides, only for maneb did we observe a significant interaction (P = 0.05); however, it was based on small numbers (two exposed individuals who grew up on farms, and six exposed individuals who did not). Women who used maneb and did not grow up on farms had an elevated risk of atopic asthma (OR, 3.97; 95% CI, 1.69–9.32). Women who used maneb and did grow up on farms had a lower risk of asthma (OR, 0.42; 95% CI, 0.10–1.73). No other pesticide exposure met our P less than 0.2 criteria.
Figure 1.
Odds ratios for cross-classifications of “grew up on a farm” with “personally applying pesticides” or “current animal exposure” and atopic asthma among farm women in the Agricultural Health Study, 1993–1997. Error bars span the 95% confidence interval.
Although we saw little evidence of an association with current animal exposures and atopic asthma using our base-model adjustments, when we included an interaction term between animal activities and “grew up on a farm,” we saw a pattern suggesting differential risk based on whether or not a woman had grown up on a farm. For all animal exposures with a history of growing up on a farm, we saw similar patterns for any animal contact (P = 0.02), beef cattle (P = 0.13), poultry (P = 0.19), and veterinary procedures (P = 0.15). This pattern was different from that for pesticide exposures among women who grew up on farms. With all the animal exposures, women who did not grow up on a farm but currently worked with animals had the highest risk of atopic asthma (OR, 1.30–1.42), whereas women who did grow up on a farm and currently worked with animals had the lowest risk (OR, 0.48–0.54). The ORs for women who grew up on farms but did not currently work with animals were less than 1.0 (OR, 0.57–0.71), but greater than the ORs for women who grew up on farms and currently worked with animals.
Because our atopic asthma group contained two different diseases (atopy and asthma), we conducted a sensitivity analysis to explore whether our results for pesticides might be due to atopy alone by removing the control subjects who had atopy from the comparison group and constructing a polytomous model with three groups: control subjects without atopy, atopy alone, and subjects with atopic asthma. Table 5 presents the ORs for atopy and atopic asthma using control subjects without atopy for the 10 pesticides significant for atopic asthma in Table 2. No other pesticides were statistically significant for atopic asthma when we removed subjects with atopy from the comparison group. For these 10 pesticides, we also observed associations for atopy alone; however, the estimates for atopy alone were all less than the corresponding estimates for atopic asthma, suggesting a gradient in the effect of these pesticides from atopy alone to atopy with asthma. Only glyphosate had ORs that were almost identical between the two groups (1.31 for atopy alone vs. 1.35 for atopic asthma). For coumaphos and phorate, the associations appeared solely related to atopic asthma, as no association was observed for atopy alone. Only for metalaxyl, however, were the ORs for atopy alone and for atopic asthma significantly different from each other (P = 0.05).
TABLE 5.
ODDS RATIOS FOR ATOPY ALONE AND ATOPIC ASTHMA COMPARED WITH CONTROL SUBJECTS WITHOUT ATOPY AMONG FARM WOMEN IN THE AGRICULTURAL HEALTH STUDY
| Patients with Asthma
|
||||||
|---|---|---|---|---|---|---|
| Control Subjects (n = 21,803)
|
Atopy Alone (n = 3,086)
|
Atopic Asthma (n = 282)
|
P Value for Difference§ | |||
| Exposure* | n (%)† | n (%)† | OR (95% CI)‡ | n (%)† | OR (95% CI)‡ | |
| Herbicides | ||||||
| 2,4-d | 3,237 (15) | 477 (16) | 1.20 (1.08–1.34) | 52 (19) | 1.57 (1.15–2.15) | 0.11 |
| Glyphosate | 7,243 (34) | 1,174 (39) | 1.31 (1.21–1.42) | 106 (39) | 1.35 (1.05–1.73) | 0.81 |
| Insecticides | ||||||
| Carbaryl | 6,604 (31) | 1,119 (37) | 1.36 (1.26–1.48) | 106 (38) | 1.46 (1.14–1.87) | 0.58 |
| Coumaphos | 281 (1) | 37 (1) | 1.00 (0.71–1.42) | 7 (3) | 2.16 (1.01–4.64) | 0.07 |
| DDT | 761 (4) | 148 (5) | 1.60 (1.33–1.93) | 16 (6) | 1.91 (1.13–3.24) | 0.53 |
| Malathion | 4,273 (20) | 698 (23) | 1.28 (1.17–1.41) | 76 (28) | 1.65 (1.26–2.16) | 0.08 |
| Parathion | 195 (1) | 44 (1) | 1.68 (1.21–2.34) | 7 (3) | 3.15 (1.46–6.79) | 0.13 |
| Permethrin (animals) | 758 (4) | 132 (4) | 1.36 (1.12–1.64) | 15 (6) | 1.77 (1.04–3.02) | 0.34 |
| Phorate | 421 (2) | 56 (2) | 1.07 (0.81–1.42) | 10 (4) | 2.06 (1.08–3.93) | 0.06 |
| Fungicides | ||||||
| Metalaxyl | 299 (1) | 64 (2) | 1.36 (1.03–1.80) | 10 (4) | 2.73 (1.41–5.29) | 0.05 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Only those pesticides significant in the original models (Table 2) are presented here.
Percentages based on numbers responding to the question; missing values are not included.
ORs adjusted for age, state, smoking status, body mass index, and “grew up on farm.”
P value for the test formally comparing the ORs for atopic and nonatopic asthma.
DISCUSSION
Among farm women in the Agricultural Health Study, growing up on a farm was protective against both atopic and nonatopic asthma, with the greater effect for atopic asthma. Despite this reduced risk, use of pesticides, particularly insecticides, was positively associated with atopic asthma. We saw weak evidence of an interaction between growing up on a farm and use of pesticides with atopic asthma. Although personally mixing or applying pesticides increased the risk of atopic asthma, regardless of whether a woman grew up on a farm or not, the increase in risk was larger among those who grew up on farms. Current farm exposures did not confound the results for pesticides. Growing up on a farm also modified the risk of atopic asthma associated with currently working with animals. Women who did not grow up on farms had higher risk if they currently worked with animals, but women who grew up on farms had a lower risk if they currently worked with animals.
Pesticides, particularly organophosphate and carbamate insecticides, may contribute to airway reactivity and asthma among farmers. Early case reports suggested an association of organophosphate insecticides with exacerbation of asthma (17, 18); however, few population-based studies have explored this association. Beard and colleagues reported excess asthma mortality among pesticide applicators, particularly among those who applied pesticides during the organophosphate era (after 1962) (3). In a cross-sectional study of 1,939 farmers in Saskatchewan, carbamate and organophosphate insecticides were associated with current asthma; the effect was greater for carbamates, particularly carbofuran (OR, 2.0; 95% CI, 1.3–3.2) (2). In the current analysis, we also saw an approximately twofold increase in atopic asthma with carbofuran (OR, 1.92; 95% CI, 0.98–3.77). Among chlorpyrifos manufacturing workers, the prevalence of asthma and other respiratory diseases (as classified by International Classification of Diseases, ninth revision [ICD-9], codes 490–496) was elevated (OR, 1.41; 95% CI, 0.95–2.09), suggesting a potential association with asthma; however, because all ICD-9 codes were combined, the increased prevalence may be associated with some other respiratory disease in this category (e.g., chronic bronchitis) (19). Pesticide application was associated with asthma symptoms among Brazilian farmers, but there was no association with organophosphate insecticides or any other specific category of pesticide (20). We observed ORs greater than 2.0 for the most potent organophosphates (parathion, phorate, and coumaphos) for atopic asthma, and a somewhat weaker association with malathion. Although we did not have information on wheeze among farm women, we previously saw increased wheeze associated with current use of specific pesticides among both the farmers (4) and commercial pesticide applicators (5) in the AHS. We saw increased wheeze with current use of specific pesticides, including coumaphos, malathion, parathion, phorate, and permethrin on animals; these same pesticides were also associated with atopic asthma among the farm women studied here. Results for current wheeze and prevalent asthma are not comparable, because the time periods of exposure differ, as do the patterns of pesticide use, between farmers and their wives (9).
Recent animal studies have demonstrated that organophosphates induce airway hyperreactivity, potentially at doses below those causing acetylcholinesterase inhibition (6, 7, 21). Pesticides may also modulate inflammatory responses to other farm exposures, such as endotoxin and allergens. For example, carbaryl, a commonly used carbamate insecticide, may enhance the allergenicity of dust mites (22). Malathion, an organophosphate insecticide, stimulated inflammatory mediators, resulting in increased macrophage function in mice (23). In rats treated with the organophosphate insecticide acephate at levels that did not inhibit acetylcholinesterase, Singh and Jiang observed significantly altered endotoxin-induced changes in the levels of proinflammatory cytokines, including tumor necrosis factor-α and inducible nitric oxide synthase mRNA (24). Rats exposed via inhalation to dichlorvos, another organophosphate insecticide, had increased production of IFN-γ, especially after adrenalectomy, suggesting a role for inflammation in the response to this insecticide (25). Specific pesticides (carbaryl, 2,4-d, alachlor, and zineb) modulated endotoxin-induced macrophage activation through inhibition of nuclear factor-κB activation (26, 27). The primary metabolite of chlorpyrifos, chlorpyrifos oxon, enhanced the endotoxin-induced IFN-γ response in human blood cell cultures, suggesting that chlorpyrifos, in combination with endotoxin, may lead to an increased type 1 immune response (28). Although limited, these data suggest that pesticides, including those associated with atopic asthma in this analysis, may interact with inflammatory pathways involving endotoxin processing.
Although atopic and nonatopic asthma may have different etiologies (13, 14, 29), we only observed significant differences between these groups for “growing up on a farm” and for pesticide use, both overall and for carbamates. Associations for other farming exposures were similar between the two groups, except for animal exposures when stratified by “growing up on a farm.” Limited animal and in vitro data suggest that pesticide exposure may alter the protective effect of early-life endotoxin (26–28). Others have observed that use of quaternary ammonia compounds as disinfectants on farms was associated with atopic sensitization in the presence of endotoxin among adult farmers, suggesting that other chemical agents besides pesticides may interact with endotoxin to contribute to allergic asthma (30). We saw a statistical interaction between pesticide use and “growing up on a farm,” but the interaction was limited to any pesticide use, and not specific chemicals (except maneb, for which the sample size was very small). We also observed that growing up on a farm confounded the pesticide results for atopic asthma; the effect estimates were lower when “growing up on a farm” was not included. This confounding could be the result of women who grew up on farms being more likely to apply pesticides than women who did not (62 vs. 50%), or it may be due to some biological interaction between early-life exposures and pesticide use.
We relied on self-reported cross-sectional data for this analysis. Self-reported doctor-diagnosed asthma is a reliable and valid endpoint (31, 32). Among farmers, subjects with self-reported asthma have a higher symptom prevalence and lower lung function than subjects without asthma (2). We studied prevalent disease information; thus, some of our cases will have been diagnosed more recently than others. We did not have information on either respiratory or allergic symptoms, or on duration of disease. To assess potential occupational associations, we limited our analysis to cases of adult-onset asthma diagnosed after age 19 years. The prevalence of adult-onset asthma in our study is similar to that reported for current asthma among California farmers (2.7%) (33) and Norwegian farmers (3.7%) (34). Atopy was defined as a history of doctor-diagnosed eczema or hay fever; we (4, 35, 36) and others (37) have previously used similar questionnaire-based definitions for atopy. We created the two case groups based on responses to three questions (asthma, eczema, and hay fever) in a manner similar to that of Upton and colleagues, who used asthma with and without hay fever to define atopic and nonatopic asthma among adults (37). Using this definition, 63% of adult subjects with asthma in Scotland in 1996 were classified as nonatopic, an estimate which is very similar to ours (60%). Among Norwegians, 70% of adult asthma was classified as nonatopic, with atopy defined by IgE (12). Our atopy definition is based on a doctor's diagnosis of hay fever or eczema; thus, we may overestimate the prevalence of nonatopic asthma if people who experience allergic symptoms do not receive a hay fever diagnosis. Because the prevalence of nonatopic asthma is similar in our sample to that in two other populations, including one with IgE definition, we feel that such overestimation, if present, is minor. A potential explanation for our differential results between atopic and nonatopic asthma could be that factors affecting risk for atopic asthma reflect primarily risk of atopy, and not asthma per se. Our sensitivity analysis in Table 5 shows that atopy alone cannot fully explain the observed differences between atopic and nonatopic asthma, because the ORs for individual pesticides were consistently larger among those with atopic asthma than those with atopy alone.
Our exposure information was based on self-reported lifetime pesticide use, both total pesticide use and use of specific pesticides. For individual pesticides, we lacked duration or frequency information and were limited in our ability to assess dose response. Farmers have been demonstrated to provide reliable information regarding their personal pesticide use (38, 39), and we believe that farm women, particularly those who apply pesticides in agricultural settings, are also likely to do so. We have no reason to believe that pesticide use reporting would differ by asthma or atopy status, and, thus, reporting errors would tend to bias our results toward the null. Because we lacked information on duration of use, we relied on ever-use of a pesticide in a woman's lifetime; thus, women with one use of a particular chemical are combined with women who may have used the chemical for decades, which may dilute the association. Recall may be better for more commonly used pesticides and more recent use, and this feature may lead to underestimates of the association with rarely or historically used chemicals. We had no data on lifetime farming exposures that may contribute to asthma; hence, the findings for some specific pesticides (e.g., coumaphos and permethrin) may be an indicator that these are surrogates for historic animal exposures. However, a majority of the pesticides associated with asthma have not been used in animal production.
Farms represent a complex occupational setting with opportunities for a number of concurrent exposures, including multiple pesticides. We adjusted the pesticide models for other correlated pesticides. Only the commonly used insecticides, malathion and carbaryl, required adjustment. The OR for malathion, but not for carbaryl, remained significantly elevated after mutual adjustment. We controlled for other potential confounders, including smoking, in our base model. To further explore whether smoking was associated with atopic and nonatopic asthma, we limited our analysis to nonsmoking farm women and observed similar associations (data not shown), but the reduced sample size impaired power.
Our study of over 25,000 farm women represents the largest sample of farm women evaluated for adult asthma. Previous work among farmers and agricultural workers has generally been limited to a few types of farming (e.g., livestock or crops), and, except for a few studies, has not had the opportunity to focus on pesticides. In our sample, 57% of farm women applied pesticides at some point in their lives. The number and type of pesticides, as well as the frequency of application, differed among women, thus providing us an opportunity to look at individual pesticides while controlling for correlated exposures. Our findings are consistent with a protective effect of early-life farm exposures on asthma, and suggest that pesticides, particularly organophosphate insecticides, may increase asthma risk.
Acknowledgments
The authors thank Stuart Long for data analysis. This work could not have been completed without the hard work of the Iowa and North Carolina Field Stations (Ellen Heywood, Chuck Lynch, Margaret Hayslip, and Charles Knott) and the AHS coordinating center (Marsha Dunn, Kate Torres, Stanley Legum). The authors express their appreciation of the AHS cohort members for their contribution to this work.
Supported by the intramural research program of the National Institutes of Health, the National Institute of Environmental Health Sciences, and the National Cancer Institute.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH.
Originally Published in Press as DOI: 10.1164/rccm.200706-821OC on October 11, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J, Douwes J, Dowling K, Enarson D, Green F, et al. Respiratory health hazards in agriculture. Am J Respir Crit Care Med 1998;158(Suppl):S1–S76. [DOI] [PubMed] [Google Scholar]
- 2.Senthilselvan A, McDuffie HH, Dosman JA. Association of asthma with use of pesticides: results of a cross-sectional survey of farmers. Am Rev Respir Dis 1992;146:884–887. [DOI] [PubMed] [Google Scholar]
- 3.Beard J, Sladden T, Morgan G, Berry G, Brooks L, McMichael A. Health impacts of pesticide exposure in a cohort of outdoor workers. Environ Health Perspect 2003;111:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppin JA, Umbach DM, London SJ, Alavanja MCR, Sandler DP. Chemical predictors of wheeze among farmer pesticide applicators in the agricultural health study. Am J Respir Crit Care Med 2002;165:683–689. [DOI] [PubMed] [Google Scholar]
- 5.Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. Am J Epidemiol 2006;163:1129–1137. [DOI] [PubMed] [Google Scholar]
- 6.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci 2005;83:166–176. [DOI] [PubMed] [Google Scholar]
- 7.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 2004;286:L963–L969. [DOI] [PubMed] [Google Scholar]
- 8.Engberg L. Women and agricultural work. Occup Med 1993;8:869–882. [PubMed] [Google Scholar]
- 9.Kirrane EF, Hoppin JA, Umbach DM, Samanic C, Sandler DP. Patterns of pesticide use and their determinants among wives of farmer pesticide applicators in the Agricultural Health Study. J Occup Environ Med 2004;46:856–865. [DOI] [PubMed] [Google Scholar]
- 10.McDuffie HH. Women at work: agriculture and pesticides. J Occup Med 1994;36:1240–1246. [DOI] [PubMed] [Google Scholar]
- 11.Karjalainen A, Kurppa K, Virtanen S, Keskinen H, Nordman H. Incidence of occupational asthma by occupation and industry in Finland. Am J Ind Med 2000;37:451–458. [DOI] [PubMed] [Google Scholar]
- 12.Eduard W, Omenaas E, Bakke PS, Douwes J, Heederik D. Atopic and non-atopic asthma in a farming and a general population. Am J Ind Med 2004;46:396–399. [DOI] [PubMed] [Google Scholar]
- 13.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med 2004;10:44–50. [DOI] [PubMed] [Google Scholar]
- 14.Jayaratnam A, Corrigan CJ, Lee TH. The continuing enigma of non-atopic asthma. Clin Exp Allergy 2005;35:835–837. [DOI] [PubMed] [Google Scholar]
- 15.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MCR, Sandler DP. Pesticide exposure and allergic and nonallergic asthma among farm women in the Agricultural Health Study. Am J Epidemiol 2006;163(Suppl 11):S157. [Google Scholar]
- 16.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, et al. The Agricultural Health Study. Environ Health Perspect 1996;104:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant DH. Asthma due to insecticide sensitivity. Aust N Z J Med 1985;15:66–68. [DOI] [PubMed] [Google Scholar]
- 18.Weiner A. Bronchial asthma due to the organic phosphate insecticides: a case report. Ann Allergy 1961;19:397–401. [PubMed] [Google Scholar]
- 19.Burns CJ, Cartmill JB, Powers BS, Lee MK. Update of the morbidity experience of employees potentially exposed to chlorpyrifos. Occup Environ Med 1998;55:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria NMX, Facchini LA, Fassa AG, Tomasi E. Pesticides and respiratory symptoms among farmers. Rev Saude Publica 2005;39:973–981. [DOI] [PubMed] [Google Scholar]
- 21.Segura P, Chavez J, Montano LM, Vargas MH, Delaunois A, Carbajal V, Gustin P. Identification of mechanisms involved in the acute airway toxicity induced by parathion. Naunyn Schmiedebergs Arch Pharmacol 1999;360:699–710. [DOI] [PubMed] [Google Scholar]
- 22.Dong W, Gilmour MI, Lambert AL, Selgrade MK. Enhanced allergic responses to house dust mite by oral exposure to carbaryl in rats. Toxicol Sci 1998;44:63–69. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers K, Xiong S. Contributions of inflammatory mast cell mediators to alterations in macrophage function after malathion administration. Int J Immunopharmacol 1997;19:149–156. [DOI] [PubMed] [Google Scholar]
- 24.Singh AK, Jiang Y. Lipopolysaccharide (LPS) induced activation of the immune system in control rats and rats chronically exposed to a low level of the organothiophosphate insecticide, acephate. Toxicol Ind Health 2003;19:93–108. [DOI] [PubMed] [Google Scholar]
- 25.Elia J, Aoki A, Maldonado CA. Regulation of uteroglobin/Clara cell protein expression after acute lung exposure to an organophosphoreted insecticide. Histochem Cell Biol 2003;120:33–39. [DOI] [PubMed] [Google Scholar]
- 26.Shimomura-Shimizu M, Sugiyama K, Muroi M, Tanamoto K. Alachlor and carbaryl suppress lipopolysaccharide-induced iNOS expression by differentially inhibiting NF-κB activation. Biochem Biophys Res Commun 2005;332:793–799. [DOI] [PubMed] [Google Scholar]
- 27.Hong CC, Shimomura-Shimizu M, Muroi M, Tanamoto K. Effect of endocrine disrupting chemicals on lipopolysaccharide-induced tumor necrosis factor-α and nitric oxide production by mouse macrophages. Biol Pharm Bull 2004;27:1136–1139. [DOI] [PubMed] [Google Scholar]
- 28.Duramad P, Tager IB, Leikauf J, Eskenazi B, Holland NT. Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1. J Appl Toxicol 2006;26:458–465. [DOI] [PubMed] [Google Scholar]
- 29.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet 2006;368:804–813. [DOI] [PubMed] [Google Scholar]
- 30.Preller L, Doekes G, Vermeulen R, Heederik D, Vogelzang PFJ, Boleij JSM. Disinfectant use as a risk factor for atopic sensitization and symptoms consistent with asthma: an epidemiological study. Eur Respir J 1996;9:1407–1413. [DOI] [PubMed] [Google Scholar]
- 31.Burney PG, Laitinen LA, Perdrizet S, Huckauf H, Tattersfield AE, Chinn S, Poisson N, Heeren A, Britton JR, Jones T. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: an international comparison. Eur Respir J 1989;2:940–945. [PubMed] [Google Scholar]
- 32.Jenkins MA, Clarke JR, Carlin JB, Robertson CF, Hopper JL, Dalton MF, Holst DP, Choi K, Giles GG. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol 1996;25:609–616. [DOI] [PubMed] [Google Scholar]
- 33.Monso E, Schenker M, Radon K, Riu E, Magarolas R, McCurdy S, Danuser B, Iversen M, Saiki C, Nowak D. Region-related risk factors for respiratory symptoms in European and Californian farmers. Eur Respir J 2003;21:323–331. [DOI] [PubMed] [Google Scholar]
- 34.Eduard W, Douwes J, Omenaas E, Heederik D. Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax 2004;59:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Diesel exhaust, solvents, and other occupational exposures as risk factors for wheeze among farmers. Am J Respir Crit Care Med 2004;169:1308–1313. [DOI] [PubMed] [Google Scholar]
- 36.Hoppin JA, Umbach DM, London SJ, Alavanja MCR, Sandler DP. Animal production and wheeze in the Agricultural Health Study: interactions with atopy, asthma, and smoking. Occup Environ Med 2003;60:3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upton MN, McConnachie A, McSharry C, Hart CL, Smith GD, Gillis CR, Watt GC. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan Family Study surveys of parents and offspring. BMJ 2000;321:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair A, Zahm SH. Patterns of pesticide use among farmers: implications for epidemiologic research. Epidemiology 1993;4:55–62. [DOI] [PubMed] [Google Scholar]
- 39.Hoppin JA, Yucel F, Dosemeci M, Sandler DP. Accuracy of self-reported pesticide use duration information from licensed pesticide applicators in the Agricultural Health Study. J Expo Anal Environ Epidemiol 2002;12:313–318. [DOI] [PubMed] [Google Scholar]