Abstract
Development of gene transfer vectors with regulated, lung-specific expression will be a useful tool for studying lung biology and developing gene therapies. In this study we constructed a series of lentiviral vectors with regulatory elements predicted to produce lung-specific transgene expression: the surfactant protein C promoter (SPC) for alveolar epithelial type II cell (AECII) expression, the Clara cell 10-kD protein (CC10) for Clara cell expression in the airway, and the Jaagskiete sheep retrovirus (JSRV) promoter for expression in both cell types. Transgene expression from the SPC and CC10 vectors was restricted to AECII and Clara cell lines, respectively, while expression from the JSRV vector was observed in multiple respiratory and nonrespiratory cell types. After intratracheal delivery of lentivector supernatant to mice, transgene expression was observed in AECII from the SPC lentivector, and in Clara cells from the CC10-promoted lentivector. Transgene expression was not detected in nonrespiratory tissues after intravenous delivery of CC10 and SPC lentiviral vectors to murine recipients. In summary, incorporation of genomic regulatory elements from the SPC and CC10 genes resulted in respiratory specific transgene expression in vitro and in vivo. These vectors will provide a useful tool for the study of lung biology and the development of gene therapies for lung disorders.
Keywords: gene therapy, lentivirus vector, regulated gene expression
CLINICAL RELEVANCE
These vectors will improve the safety of lung gene therapy applications. They will also be useful to basic scientists, to direct lung-specific expression of genes and/or track the progeny of transduced stem cells.
Gene therapy has been proposed as a treatment for single gene inherited and acute lung disorders. Two components of successful long-term gene therapy for lung disorders are the efficient transfer of genes to a self-renewing target cell population and regulated transgene expression in the desired differentiated cell populations. Transient gene transfer to the respiratory epithelium has been demonstrated from a variety of viral and nonviral vectors gene transfer systems in animal studies (1). For example, recombinant adenoviral gene transfer systems have been evaluated for gene transfer to the lung because of their natural tropism for the respiratory tract. However, adenoviral vectors do not efficiently infect the respiratory epithelium in large part because the coxsackievirus and adenovirus receptor (CAR) is located on the basolateral surface (2). Furthermore, adenoviral vectors induce potent immune responses to viral genes encoded on the vectors, contaminating helper virus and/or capsid proteins, which in turn can result in the elimination of transduced cells. Many groups have modified adenoviral vectors to reduce or prevent immune responses; however, these vectors generally have a reduced infection rate (3, 4). Regardless of the adenovirus configuration, adenoviral vectors do not integrate into the host genome and are lost from cycling target cells within days or weeks of transduction, thus limiting their use for long-term gene therapies and studies.
Vectors based on the nonpathogenic human parvovirus, adenoassociated virus (AAV), are also being evaluated for gene delivery in the lung (5). Recombinant AAV vectors can integrate into cells, but are more often maintained as an episome that is diluted or lost with cell division (5, 6). Furthermore, their small insert size makes it difficult to produce recombinant vectors with large transcriptional units such as an epithelial-specific promoter expressing the cystic fibrosis transmembrane regulator, which can be 8 kb or more in length.
Nonviral gene transfer systems using liposomes or naked DNA have also been used to transfer genes to the respiratory epithelium (7, 8). The advantages of these systems are that transgenic cassettes can be transferred without viral sequences, and that large or multiple genes can be transferred regardless of the proliferative status and cell surface receptor profile of the target cell. However, the overall efficiency of gene transfer is low, genes rarely integrate, and the vectors are lost in multiplying cells, and thus this approach is not useful to achieve long-term stable gene therapy.
For long-term gene therapies or biological studies, a vector that can integrate efficiently into the target cell genome is required (9). Type “C” retroviruses such as Moloney murine leukemia virus, or lentiviruses such as HIV-1, stably integrate into the genome of the target cell. Deletion of the coding sequences for viral gene products renders the gene transfer vectors replication incompetent and makes 6–8 kb available for the insertion of desired genes (10). Lentiviral gene transfer vectors can transduce and integrate into nondividing cells; however, they generally transduce dividing cells more efficiently (11–13).
Lentiviral gene transfer vectors, such as the CCL vector developed by Zufferey and colleagues contain ∼ 10% of the wild-type HIV-1 genome (14). Recombinant lentiviral vectors pseudotyped with the vesicular stomatitis G protein (VSV-G) can transduce a wide variety of nondividing cells. Several studies have demonstrated lentiviral gene transfer of HIV-1– or FIV-based vectors to respiratory epithelium in quiescent, polarized epithelial cultures in vitro (15, 16) or nasal, airway, bronchial, and alveolar epithelial cells in vivo (16–20). Transduced cells can be detected for long periods of time in vivo, suggesting that these vectors can integrate into either long-lived cells, or respiratory progenitors, which contribute progeny for long periods of time.
A second requirement for gene therapy is the consistent and lineage-regulated transgene expression. While constitutive transgene expression may be suitable for gene therapy applications in deficiencies of housekeeping genes, such as lysosomal storage disease or other enzyme deficiencies, it will not be acceptable for other disorders requiring regulation of transgene expression. This is particularly important after the development of leukemias in X-SCID patients receiving hematopoietic cells retrovirally transduced with the common γ chain cDNA, due to the disregulation of proto-oncogenes in the genome from the strong retroviral enhancers from the integrated vector (21).
For lineage-restricted transgene expression from lentiviral vectors, a self-inactivating vector design in which the viral promoter and enhancer in the U3 region of the 3′ long terminal repeat (LTR) are removed from the vector plasmid can be used. During integration, the U3 region of the 3′LTR is copied to the 5′LTR, thereby resulting in the elimination of the 5′ viral promoter in the integrated provirus (14, 22). The transgene can be expressed from an internal lineage-specific or regulated promoter/regulatory elements. Lentiviral vectors have been used for regulated transgene expression in a variety of lineages such as B lymphocytes (23), T lymphocytes (24, 25), or erythrocytes (26, 27); however, they have not been described for respiratory cell types.
In these studies we describe the development and evaluation of a series of lentiviral vectors in which an enhanced green fluorescent protein (EGFP) marker transgene is expressed from promoters and regulatory elements predicted to provide airway and/or alveolar epithelial specific expression. The cellular promoters evaluated in this study are from the surfactant protein C (SPC) (28) or the Clara cell 10-kD secretory protein (CC10) (29) genes to provide alveolar epithelial type II (AECII) or Clara cell restricted expression, respectively. The promoter from the Jaagskiete sheep retrovirus (JSRV), which is predicted to express in both AECII and Clara cells (30), was also evaluated. The lineage profile of transgene expression from lentivectors with each of these promoters was evaluated in cell lines in vitro and in lungs after in vivo delivery of viral supernatant to mice. This study describes the development of lentivirus vectors with respiratory epithelial-specific transgene expression, which will facilitate the development of lung gene therapies and the study of lung development and biology.
MATERIALS AND METHODS
Vector Construction and Supernatant Production
The lentiviral vector backbone used in this study was the pCCL self-inactivating vector kindly provided by Dr. Luigi Naldini (CellGenesys, San Francisco, CA) (14). In this backbone, the viral enhancers and TATA box in the U3 promoter region of the 3′LTR have been deleted. This results in a crippling of the promoter in the integrated provirus, as the U3 region of the 3′LTR is copied to the 5′LTR during integration.
The basic vector backbone, CCL-X-EGFP, contains a multicloning site (X) upstream of the EGFP reporter gene into which the test promoters could be inserted. This vector backbone also served as a “no-promoter” negative control. The human SPC promoter was investigated for AECII-specific expression (28). The rat CC10 promoter was evaluated for airway specific expression in Clara cells (29). The CC10 and SPC promoters were from Dr. Jeffrey Whitsett (Cincinnati Children's Hospital Medical Center, Cincinnati, OH). For expression in both AECII and Clara cells we evaluated the promoter from the U3 region of the JSRV from Dr. Hung Fan (University of California, Irvine, CA) was evaluated. The lentivector with the constitutive promoter from the retroviral MND vector LTR (31) promoter/enhancer was provided by Dr. Donald B. Kohn (Childrens Hospital Los Angeles, Los Angeles, CA) and used as positive control vectors in these studies.
VSV-G pseudotyped lentiviral supernatants were produced by triple transfection of the human embryonic kidney 293T cell line as previously described (23). In brief, 5 × 106 293T cells were seeded in a poly-L-lysine (Sigma-Aldrich, St. Louis, MO)–treated 10-cm tissue culture dishes overnight. The following day, the cells were transfected with 10 μg of the vector plasmid, 10 μg of the 8.9 packaging plasmid (10) (which does not express HIV-1 accessory proteins), and 2 μg of pMDG-VSV-G plasmid using a standard calcium phosphate transfection. For larger viral supernatant preparations, all components of the transfection culture were scaled up by one log for 500 cm2 tissue culture plates (Costar, Lowell, MA). Sixteen hours later the medium was removed, and the cells were washed with PBS and then exposed to 10 mM sodium butyrate (Sigma-Aldrich) for 8 hours. Supernatant was collected at 72 to 96 hours after transfection and concentrated by centrifugation at 25,000 rpm for 90 minutes in a Beckman coulter: Optima L-90K Preparative Ultracentrifuge in a swing bucket rotor (SW32Ti) (Beckman Coulter, Fullerton, CA). Since the promoters from all viral vectors would not express in a single cell type, raw titers were determined after the addition of serial dilutions of viral supernatant supplemented with 8 μg/ml polybrene (Sigma-Aldrich) to a cell type which expresses each promoter. The SPC and JSRV vectors were titered on MLE-12 or MLE-15 cells and the CC10 vector on H441. The raw titers were calculated by:
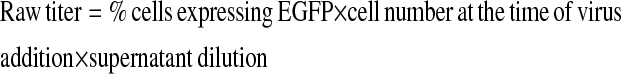 |
Since the transduction efficiency was not equivalent between all cell lines, each cell line was also simultaneously transduced with the positive control CCL-MND-EGFP, to normalize the titer to the efficiency of gene transfer on 293A cells (Invitrogen, Carlsbad, CA). The normalized titers were used to calculate the concentration of virus, and to make dilutions for each experiment.
Cell Line Transduction and Flow Cytometry Analysis
For transduction experiments, 1 × 105 cells were plated in each well of a 6-well tissue culture treated plate. The following day, the medium was replaced with lentiviral supernatant and supplemented with 8 μg/ml of polybrene. The vector supernatant medium was replaced the following morning with fresh growth medium. The cells were passaged as needed and the proportion of cells expressing EGFP was determined by flow cytometry 5 to 7 days later.
For flow cytometry analysis of EGFP transgene expression, cells were trypsinized, washed in PBS, and resuspended in indicator-free Hanks' buffered saline (Invitrogen). The samples were analyzed on a FACs Calibur using Cellquest software (Becton Dickson, San Jose, CA).
Animal Husbandry and Tissue Collection
Timed pregnant C57B6J female mice were purchased from Jackson Laboratories (Bar Harbor, ME), and the 6- to 8-wk-old athymic nudes were purchased from Harlan Sprague Dawley (Indianapolis, IN). All mice were maintained at the Childrens Hospital Los Angeles' Saban Research Institute's Central Animal Facility for the duration of study. All studies were approved by the Institutional Animal Care and Use Committee at Childrens Hospital Los Angeles.
At the time of killing, mice were anesthetized by intraperitoneal delivery of sodium pentobarbital (150 mg/kg). The thoracic and abdominal cavities were surgically opened, the renal artery cut, and the animal was perfused with at least 10 ml of PBS through the right ventricle of the heart. The lungs were inflated with Tissue-Tek OCT compound (Fisher Scientific, Tustin, CA) through a 22-gauge intravenous catheter inserted into the trachea. Lung, trachea, heart, thymus, kidney, intestine, liver, spleen, and skeletal muscle were harvested for EGFP immunofluorescence and proviral DNA analysis. For immunofluorescence analysis pieces of tissue were embedded in OCT medium and stored at −80°C until sectioning and immunofluorescence analysis for EGFP expression as described below. Aliquots of tissue were also collected for proviral DNA analysis by snap-freezing in liquid nitrogen and storage at −80°C until extraction.
In Vivo Delivery of Lentivirus Supernatant
To evaluate the lineage profile of transgene expression in primary tissues, mice were injected with lentiviral supernatant either intravenously or intratracheally. Intravenous administration was performed as described (32). In brief, each Day 0 neonatal C57B6J pup received 1 × 108 international units (iu) diluted to 50 μl with sterile preservative-free saline and 8 μg/ml polybrene delivered via the facial vein. The pups weighed from 1.3 to 1.8 g at the time of injection, resulting in a vector dose of 5.5 to 7.7 × 107 iu/g. After the injections, the pups were returned to their nest, nursed, and weaned following standard protocols.
For intratracheal delivery of lentivirus/keratinocyte growth factor (KGF) mixture, anesthesia was induced with 5% isofluorane and maintained with 2.5% isofluorane in 6- to 8-wk-old athymic nude mice (Harlan, Indianapolis, IN). The neck was extended and cleaned with a chlorhexidine solution. A small midline incision was made with a sterile scalpel to expose the trachea. A total volume of 50 μl containing 1 × 108 vector particles (suspended in 0.9% saline with 5 mg/kg recombinant human [rh] KGF) was injected into the trachea using a 0.5-cc Insulin syringe with 28-Ga needle (Becton-Dickson). Control mice received intratracheal delivery of 50 μl saline mixed with 5 mg/kg rhKGF. After injection, the wound was treated with 1 to 2 drops of bupivicane (as a local anesthetic) and closed with a tissue adhesive. The mice were given antibiotics (Maxim-200 μg/ml in sterile water) for 1 month and ketoprofen subcutaneously on the day of surgery, and the day after surgery. The mice were killed at 1 to 6 weeks after injection for EGFP expression analysis.
Immunofluorescence Staining of Tissue Sections
Frozen lungs were sectioned at 5 μm on a CM1900 cryostat (Leica, Bannockburn, IL) and placed on a Plus glass slide (Fisher Scientific, Tustin, CA). Dried sections were fixed with pre-cooled (−20°C) 100% acetone for 10 minutes at room temperature. The sections were stained to localize AECII and Clara cells within the tissues and evaluate which lineages were expressing EGFP. Sections were blocked with 5% normal donkey serum containing 1% bovine serum albumin, 0.1% triton X-100 and 0.05% Tween 20 in PBS for 2 hours at room temperature before applying primary antibody. Samples to be stained with antibodies directed against CD45 were blocked with the same buffer, without the addition of Triton X-100.
To identify EGFP-expressing AECII cells, lung sections were double labeled with goat anti-GFP (1:800; Abcam, Cambridge, MA) and rabbit anti-human pro-SPC antibody, which cross-reacts with mouse pro-SPC (1:1,000; Chemicon, Temecula, CA) with dilutions made in serum blocking buffer. Negative controls included sections treated the same as the labeled samples except for being incubated in blocking buffer without primary antibodies. The sections were washed in 0.05% Tween 20 in PBS (PBST), and immunoreactive cells were identified after a 1-hour incubation at room temperature with donkey anti-rabbit-IgG CY3 and donkey anti-goat IgG fluorescein isothiocyanate (FITC) (all secondary antibodies from Jackson ImmunoReseach Laboratories, West Grove, PA). The sections were washed again with PBST and the nucleus stained with DAPI (Molecular Probes, Eugene, OR). After a final wash with one change of PBST, and one wash in PBS, the sections were coverslipped with Prolong Gold Antifade Reagents (Molecular Probes), and either analyzed immediately or stored at −20°C.
To further characterize the lineages of GFP-positive cells in the alveolar tissue sections were stained with the same technique described above, but triple-labeled with: goat anti-GFP (1:800), rat anti-mouse CD45 (1:100; Caltag Laboratories, Carlsbad, CA), and rabbit anti–pro-SPC (1:1,000). Donkey anti-goat IgG FITC, donkey anti-rat IgG CY3, and donkey anti-rabbit IgG CY5 were used as secondary antibodies.
For the identification of EGFP-positive Clara cells in the airways, lung sections were incubated with a goat anti-CC10 antibody for CC10 (1:1,500; Santa Cruz Biotechnology, Santa Cruz, CA) and chicken anti-GFP (1:2,000; Abcam) overnight at 4°C. Immunoreactive cells were identified after labeling with donkey anti-goat IgG, CY3-conjugated antibody, and donkey anti-chicken FITC-conjugated antibody for 1 hour at room temperature.
Negative controls were used with every experiment, and included negative control mice treated with PBS, as well as sections from each control and experimental animal incubated with buffer without a primary antibody. All samples were visualized through a fluorescent compound microscope (Leica DMRXA) and images were captured with a digital camera (Spectra cube), and acquired using EasyFISH software (Applied Spectral Imaging, Preston, UK).
Analysis for Proviral DNA Sequences
DNA was extracted using the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN) using the manufacturer's recommended protocols. Standard PCR analysis for proviral EGFP and β-actin genomic control DNA sequences was performed on transduced cell lines and tissues from in vivo supernatant treated animals to confirm that they contained proviral sequences as described (33). The average vector copy number was determined in available DNA samples by quantitative real-time polymerase chain reaction (PCR) analysis, by comparison to serial logarithmic dilutions of genomic DNA from a Jurkat clone carrying a single copy per cell as described (33).
RESULTS
Construction and Titering of Lentiviral Vectors with Tissue-Specific Promoters
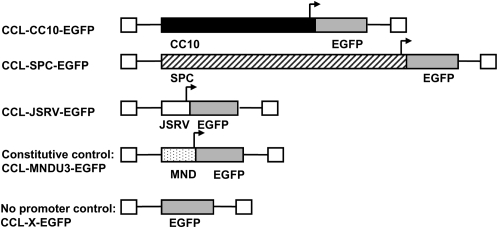
A series of lentiviral vectors were constructed in which the EGFP transgene was expressed from either an internal SPC, CC10, or JSRV promoter. Transgene expression from these vectors was compared with a positive control vector with the constitutive promoter from the U3 region of the MND retrovirus LTR (31). A no promoter control served as a negative control for cell line experiments. Schematic diagrams of each vector are shown in Figure 1. The vectors were packaged into recombinant lentiviral particles with the VSV-G protein as the envelope to transduce a wide variety of cell types. Since there was not a single cell line that would express all of the promoters evaluated in this study, titers were calculated after transduction of each vector into a cell line known to express it as described above.
Figure 1.
Schematic diagrams of integrated proviruses. The vectors are third generation self-inactivating lentiviral vectors based on the CCL series of vectors. Arrows represent a transcriptional promoter.
Evaluation of EGFP Transgene Expression in Cell Lines
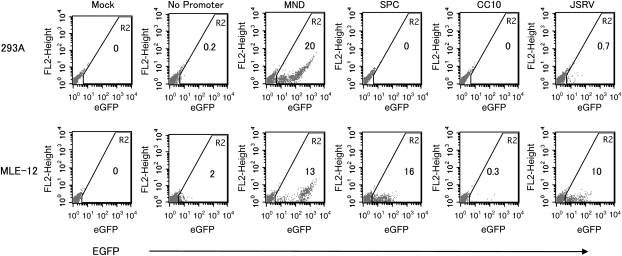
In the first set of experiments, transgene expression was evaluated from each of the test and control vectors in cell lines representing a variety of cell types. Aliquots from each cell line were exposed to an equivalent concentration viral particles from each test and control vector. Adherent cell lines were transduced with 3 × 104 iu/ml (multiplicity of infection [MOI]: 0.3) and suspension cells were transduced with 1 × 104 iu/ml (MOI: 0.1). These viral concentrations were chosen as experiments by our laboratory, and others (23) had demonstrated that these conditions minimized the proportion of cells with multiple copies of the vector, and thereby facilitated expression analysis on cells carrying a single copy of the provirus. A sample flow cytometry analysis for AECII cell line (MLE-12) and one nonrespiratory cell line (293A) is shown in Figure 2. Since not all cell lines have the same overall transduction efficiency, the data are presented as the relative proportion of cells expressing EGFP from the test vector to the constitutive MND control vector for that specific cell line. For example, a test vector expressing from the same proportion of cells as the positive control MND promoted vector in a particular cell line has an expression ratio of 1 (Figure 3).
Figure 2.
Flow cytometry analysis for EGFP transgene expression in 293A (top panel) and MLE-12 (lower panel) cell lines transduced with the panel of lentiviral vectors. Cells were transduced once with lentiviral supernatant and evaluated by flow cytometry 5 to 7 days later. The number in region 2 (R2) indicates the percentage of cells positive for EGFP.
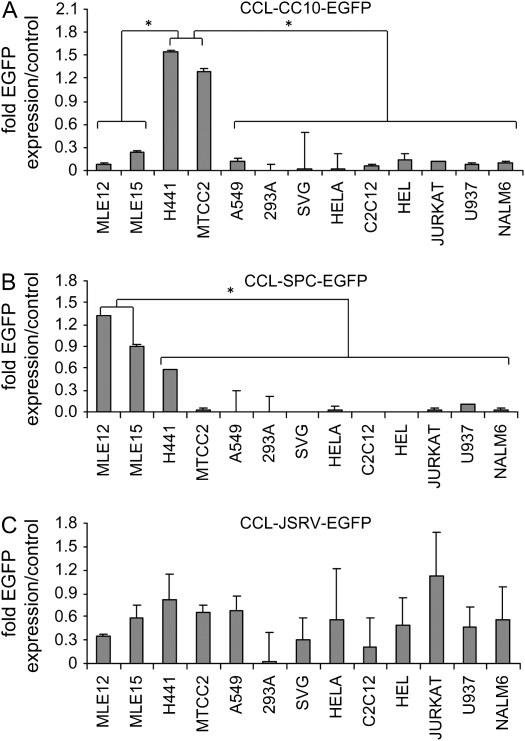
Figure 3.
Summary of transgene expression in a panel of cell lines from the (A) CCL-CC10-EGFP, (B) CCL-SPC-EGFP, and (C) CCL-JSRV-EGFP vectors. The expression levels are presented as normalized to the expression from the positive control vector CCL-MND-EGFP ± the standard error of the mean. Asterisks indicate statistically significant different levels of expression between the groups of cells with P values of < 0.05 (two-Tailed t test) with specific t test statistic values shown in the text.
The highest EGFP expression from CC10 promoter was observed in the Clara cell lines Mtcc1–2 and H441. The level of expression was higher than the CCL-MND-EGFP–positive control vector with average EGFP expressions of 1.3 and 1.5 for Mtcc1–2 and H441 cells, respectively, relative to the expression from MND vector, which was set at 1 (n = 4). Transgene expression was significantly lower in the other, non-Clara cell, respiratory epithelial cell lines, MLE-12 (0.09; P < 0.01), MLE-15 (0.2; P < 0.01), and A549 (0.1; P < 0.01) using two-tailed t tests (Figure 3A). The level of EGFP expression from the CC10 promoter in all nonrespiratory cell lines was also significantly lower, with a range of 0.001 to 0.14 (all P < 0.001; two-tailed t test). These data indicate that the transgene expression from the CCL-CC10-EGFP vector is regulated, and lineage-specific for Clara cell lines.
As expected the highest percentage of cells expressing EGFP from the SPC promoter was observed in AECII cell lines, which express the endogenous SPC gene (Figure 3B; n = 4). For example, the relative EGFP expression levels in the SPC-positive cell lines MLE-12 and MLE-15 were 1.3 and 0.9 relative to the percentage of cells expressing from the MND promoter (set at 1.0). The relative percentage of cells expressing EGFP in non-AECII lines was statistically lower than the expression in AECII cell lines. Specifically, Clara cell lines had relative expressions of 0.6 for H441 and 0.03 for Mtcc1–2 respectively, which were significantly lower than the average expressions of MLE-12 (P < 0.001; two-tailed t test) and MLE-15 (P < 0.001; two-tailed t test).
The relative EGFP expression from the CCL-SPC-EGFP vector in all nonrespiratory epithelial cell lines was less than 0.10 (Figure 3B). Statistical analysis demonstrated that there was a significant difference between EGFP expression in AECII cell lines MLE-12 and MLE-15 compared with all nonrespiratory cell lines (all P < 0.002, two-tailed t test). Collectively, these data demonstrate that proviral EGFP expression from the SPC promoter is highly specific to SPC-positive AECII cell lines.
In contrast to the expression from the SPC and CC10 lentivectors, moderate to high levels of transgene expression were observed from the CCL-JSRV-EGFP vector in all respiratory and nonrespiratory cell lines tested, except 293A, which had very low expression (Figure 3C; n = 4). The highest level of expression was observed in Jurkat cells (T-lymphoid) with EGFP expression ratio of 1.1, with moderate levels of expression observed in all respiratory epithelial cell lines (range: 0.4–0.8). Moderate to low levels of expression were detected in cell lines of muscle, neural, kidney, and hematopoietic origins (range, 0.03–0.6). There was no significant difference between the EGFP expression between any of the respiratory epithelial cell lines and nonrespiratory cell lines (all P values > 0.05; two-tailed t test), indicating that the CCL-JSRV-EGFP vector did not produce respiratory epithelial specific transgene expression.
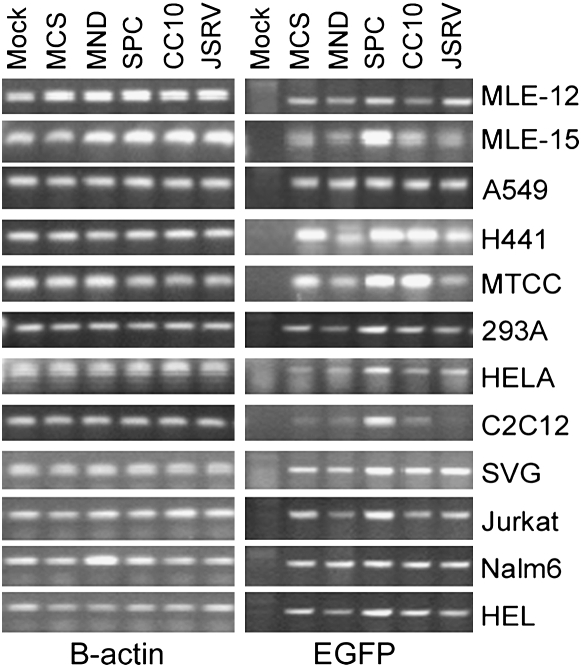
To confirm that a lack of transgene expression from a promoter was not due to a lack of proviral transduction in these experiments, all samples were evaluated by PCR analysis for proviral EGFP sequences. The data from a representative PCR analysis is shown in Figure 4, demonstrating the presence of proviral EGFP sequences in all transduced cell lines. This confirms that a lack of EGFP expression observed in nonrespiratory cell lines from the SPC and CC10 promoters was not due to a lack of gene transfer, but rather due to differences in the regulation of transgene expression in different cell types.
Figure 4.
PCR analysis for proviral EGFP (right panel) and internal control B-actin (left panel) gene sequences on DNA extracted from cells transduced with the panel of lentiviral vectors. Mock samples were not exposed to lentiviral supernatant, and no promoter samples were exposed to a control lentivirus supernatant not containing a promoter for the EGFP cDNA.
EGFP Expression in Respiratory Tissues after In Vivo Administration of CC10-EGFP Vector
To evaluate transgene expression from the CC10 and SPC lentivectors in primary respiratory epithelium, lentiviral supernatants were injected directly into the tracheas of recipient athymic nude mice. Nude mice were used in these studies to facilitate cleaning the neck region for surgery and visualizing the trachea for injection. In preliminary studies, mice were administered lentiviral supernatant intratracheally without KGF treatment; however, no transduction was observed (data not shown). While lentiviral vectors can transduce nondividing cell types, higher efficiency of transduction has been demonstrated in several cell types, with the induction of cell cycling (11–13). In all intratracheal delivery experiments presented here the mice were treated with KGF before, and at the time of, viral administration to increase Clara cell (34) and AECII (35) cell cycling. This strategy has been shown to increase cell cycling and retroviral gene transfer to the lungs in other studies (36, 37). The lungs and airways of recipient mice were harvested and evaluated at 6 weeks after vector supernatant delivery for EGFP expression by immunofluorescence analysis.
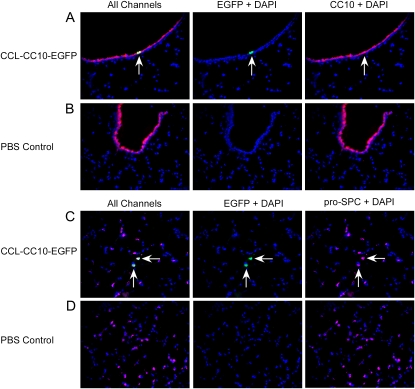
Tissue sections from recipients of the CCL-CC10-EGFP lentiviral vector were immunostained with anti-GFP antibodies. EGFP-immunoreactive cells were detected in the airways of CCL-CC10-EGFP–treated animals, with a few airway cells positive for EGFP immunofluorescence per view. EGFP-positive cells were not detected in negative control mice receiving PBS. To identify the lineage of EGFP-positive cells in the airways of experimental mice, tissue sections were labeled with antibodies directed against CC10 (red-Cy3 conjugate) and EGFP (green-FITC conjugate) proteins (Figure 5). This analysis demonstrated that the cells immunostained for EGFP (green) in the airways were also immunostained with anti-CC10 antibodies (red). Photomicrographs of representative double immunofluorescence analysis on airway sections from CCL-CC10-EGFP and PBS control animals are shown in Figures 5A and 5B, respectively. The left-hand panels show merged images with EGFP (green), CC10 (red), and DAPI-stained nuclei (blue). The middle panels show the DAPI and EGFP (green) channels, and the right panel shows only the DAPI and CC10 channels.
Figure 5.
Immunofluorescence analysis of lungs after intratracheal delivery of CCL-CC10-EGFP lentiviral supernatant (A and C) or PBS (B and D) to mice. A and B are cryosections of airway tissue immunostained for EGFP (green) and CC10 (red) with DAPI-stained nuclei (blue). C and D show cryosections of alveolar tissue immunostained for EGFP (green), pro-SP-C (pink), and DAPI-stained nuclei. The images are shown with all colors (All Channels) merged on the left panel, and with each single immunostained color with DAPI to the right of the merged image. Arrows indicate double positive cells. Original magnification of all photomicrographs: ×200.
We next determined the proportion of airway cells that were positive for EGFP by counting the total number of airway cells and the EGFP-positive airway cells in tissue sections from two recipients at 6 weeks after injection. In one recipient (IT1–8), 2.4% (10/422) of the airway cells were positive for EGFP, while in the second recipient (IT4–11) 3.6% (18/496 cells) of airway cells were positive for EGFP expression (Table 1).
TABLE 1.
SUMMARY OF THE PROPORTION OF CELLS EGFP-POSITIVE IN AIRWAY AND ALVEOLAR TISSUES AFTER INTRATRACHEAL ADMINISTRATION OF LENTIVIRAL SUPERNATANT
| EGFP Analysis of Airway Cells
|
EGFP Analysis of All Alveolar Cells
|
|||||
|---|---|---|---|---|---|---|
| Animal | Vector | Cells Counted* | Number of GFP+CC10+ | Cells Counted† | Number of GFP+proSPC+ | % GFP+ Cells |
| IT1-8 | CC10 | 422 | 10 | 2.4 | ||
| IT4-11 | CC10 | 496 | 18 | 3.6 | ||
| IT2-2 | SPC | 638 | 16 | 2.5 | ||
| IT2-5 | SPC | 966 | 22 | 2.3 | ||
| IT3-2 | SPC | 3,914 | 178 | 4.6 | ||
Definition of abbreviations: CC10, Clara cell 10-kD protein; EGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; SPC, surfactant protein C.
Exclusively airway cells were counted for this analysis.
All cells within in the alveolar region were counted for this analysis, and thus includes AECI, AECII, as well as vascular and hematopoietic cells.
In recipients of the CCL-CC10-EGFP lentivirus, alveolar cells staining positively for EGFP were also detected. To identify the lineages of the alveolar cells expressing EGFP in these recipients, cryosections were triple labeled with antibodies against EGFP (green-FITC conjugated), CC10 for Clara cells (red-cy3 conjugated), and pro-SPC for AECII (pink-Cy5 conjugated). Alveolar cells immunostaining for EGFP were also immunoreactive for pro-SPC, but negative for CC10 expression, indicating that the alveolar cells expressing from the CCL-CC10-EGFP vector were AECII (Figure 5C). This suggests that the proviral CC10 promoter was active in pro-SPC–positive cells in vivo. Cells immunoreactive for EGFP were not detected in the alveolar tissue of PBS-treated control mice (Figure 5D). These studies demonstrate that lentiviral vectors carrying the CC10 promoter direct transgene expression to both AECII in the alveolar tissue and Clara cells in the airway tissue in vivo.
EGFP Expression in Respiratory Tissues after In Vivo Administration of SPC-EGFP Vector
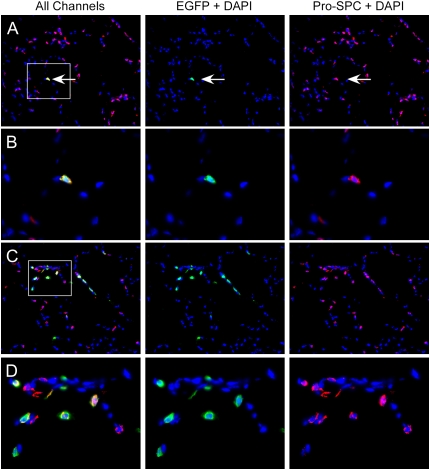
In recipients of intratracheal CCL-SPC-EGFP vector supernatant, cells immunoreactive for both EGFP and pro-SPC were observed in the alveolar tissue at all time points evaluated. The morphology, localization, and pro-SPC punctate staining pattern of these double EGFP and pro-SPC–positive cells were consistent with AECII. Figure 6 shows a photomicrograph of alveolar tissue (Figure 6A; ×200), and a high-magnification image of a double pro-SPC and EGFP-positive cell (Figure 6B; ×640), indicating that AECII cells were able to express EGFP from the lentiviral SPC promoter. The proportion of EGFP-positive cells varied between animals, tissue sections, and within an area of each tissue section, with positive cells tending to cluster within an area. Figure 6C shows an area with several EGFP-positive AECII cells clustered (×200), and Figure 6D shows a high-magnification photomicrograph of the same area (×640). To quantify the average transduction efficiency, we determined the proportion of EGFP-positive pro-SPC–positive alveolar cells in sections from three recipients at 6 weeks after injection. The proportion of all alveolar cells that were positive for both EGFP and pro-SPC ranged between 2.3% and 4.6% (Table 1). However, since the total number of alveolar cells includes a variety of alveolar cell types that are not expected to express from the SPC promoter, these percentages likely under-represent the actual proportion of transduced AECII that are expressing the EGFP transgene.
Figure 6.
Immunofluorescence analysis of lung tissue for EGFP transgene expression after intratracheal delivery of CCL-SPC-EGFP lentiviral supernatant to mice. Cryosections were immunostained for EGFP (green) and pro-SPC (red) and nuclei were counterstained with DAPI (blue). In A, the arrow shows a cell double positive for pro-SPC and EGFP immunostaining (×200). B is a higher magnification (×640) of the boxed area from A. C shows an example of an alveolar region that had a high level of AECII transduction and EGFP expression (×200). D shows a higher magnification of the boxed area in C (×640). All images are shown with all colors merged on the left panel (All Channels), and with each single immunostained color with DAPI to the right of the merged image.
At early time points in some animals, EGFP immunoreactivity was also detected in alveolar cells negative for pro-SPC staining, indicating that they were not AECII. These pro-SPC–negative EGFP-positive cells were only observed at early time points (1 and 2 wk) and not observed at the later time point (6 wk). The lineage of these pro-SPC–negative cells was identified as hematopoietic (CD45-positive) after staining for EGFP, pro-SPC, and CD45 (data not shown). EGFP-positive hematopoietic (CD45-positive) cells were not observed at the 6-week time point in any recipient. Since macrophages ingest debris, it is unclear whether the alveolar macrophages were transduced with lentivirus and expressing EGFP from the SPC promoter, or were ingesting EGFP present in the viral preparation and/or produced by transduced AECII cells. Given our in vitro data demonstrating that the CCL-SPC-EGFP vector does not express in hematopoietic cell lines (Figure 3), a lack of expression in primary transduced bone marrow (data not shown), and the fact that the EGFP was not detected at the 6-week time point, it is likely that the alveolar macrophages may be ingesting debris in the viral preparation. However, the current studies could not exclude the possibility that alveolar macrophages were expressing the transgene.
Evaluation of EGFP Expression in Nonrespiratory Tissues
In the next sets of experiments, the lineage restriction of the lentiviral vectors was evaluated after intravenous administration of lentiviral supernatant through the facial vein of Day 0 neonatal C57B6J mice. Each neonatal recipient mouse was injected with ∼ 1 × 108 viral particles of CCL-SPC-EGFP or CCL-CC10-EGFP lentiviral supernatant. Tissues were harvested from recipient mice at up to 14 months of age and aliquots stored for DNA extraction and proviral EGFP PCR analysis, and cryopreservation in OCT compound for immunostaining and fluorescence microscopy analysis for EGFP. GFP transgenic and wild-type C57B6J mice were used as positive and negative controls for these assays, respectively.
In the seven recipients of the CCL-CC10-EGFP supernatant, proviral sequences were detected in all livers, and the majority of spleens, hearts, and kidneys from neonatal recipients (Table 2). Subsequently, the level of transduction was evaluated by quantitative real-time PCR analysis on available samples. The proviral copy number in the tissues ranges from 0.060 to 0.175 copies per cell on average in the liver (the highest). Lower copy numbers were observed in the heart (range, 0.00025–0.09), spleen (range, 0.027–0.030), and kidneys (range, 0.00002–0.00006). The level of gene transfer observed in these studies is in accordance with the levels of transduction observed in other studies of lentiviral supernatant injection into neonates (32). All of the liver, heart, spleen, and kidney tissues that were positive for proviral sequences in either assay were subject to EGFP immunofluorescence microscopy analysis. Two well-spaced tissue sections were evaluated for each tissue, and EGFP expression was not detected in any of the sections analyzed (data not shown). Given copy numbers of up to 0.175 (corresponding to up to 17.5% of cells carrying a single proviral genome), these data support the in vitro cell line data demonstrating that transgene expression is not observed in several nonrespiratory tissues from the CCL-CC10-EGFP vector.
TABLE 2.
SUMMARY OF STANDARD PROVIRAL EGFP PCR ANALYSIS IN TISSUES FROM MICE INJECTED INTRAVENOUSLY WITH LENTIVIRAL SUPERNATANT
| Mouse | Vector | Kidney | Liver | Heart | Spleen | Lung |
|---|---|---|---|---|---|---|
| IV3-1 | SPC | — | + | + | + | NA |
| IV3-2 | SPC | — | + | + | + | NA |
| IV3-3 | SPC | — | + | + | + | — |
| IV3-4 | SPC | + | + | — | + | — |
| IV4-1 | CC10 | — | + | + | + | — |
| IV4-2 | CC10 | — | + | — | + | — |
| IV4-3 | CC10 | + | + | + | — | + |
| IV4-4 | CC10 | + | + | + | + | + |
| IV4-5 | CC10 | — | + | + | + | + |
| IV4-6 | CC10 | + | + | + | + | + |
| IV4-7 | CC10 | + | + | + | + | + |
| Control | PBS | — | — | — | — | — |
| Control | PBS | — | — | — | — | — |
Definition of abbreviations: +, EGFP positive; —, no EGFP detected; CC10, Clara cell 10-kD protein; NA, sample not available; SPC, surfactant protein C.
In the four intravenous recipients of the CCL-SPC-EGFP vector, all DNA samples from the spleen and liver, three of the four heart, and one of four kidney samples were positive for proviral EGFP sequences (Table 2). Quantitative PCR analysis was performed on available DNA samples. Average proviral copy numbers ranged between 0.003 and 0.09 for the heart (three samples), 0.01 and 0.025 for the spleen (three samples), and 0.03 for the kidney (one sample). Two tissue sections from all provirus-positive tissues were evaluated for EGFP expression. All tissue sections were negative for EGFP expression by immunofluorescence analysis (data not shown). These data demonstrated a variation in the level of gene transfer to nonrespiratory tissues using the neonatal intravenous approach. However, the lack of transgene expression observed in any nonrespiratory tissue supports the in vitro data, indicating that the CCL-SPC-EGFP lentivector is not expressed in nonrespiratory tissues. Collectively, the in vitro and in vivo data presented above indicate that these lentivectors produce regulated, respiratory-specific transgene expression.
DISCUSSION
Lineage-regulated transgene expression will be necessary for effective, long-term gene therapies for disorders affecting the lung. Most gene therapy clinical trials directed to respiratory epithelium to date have used constitutive promoters such as the promoter from the cytomegalovirus (CMV) or the promoter in the retroviral LTR. However, long-term animal studies have indicated that, over time, transgene expression can be silenced from viral promoters (38, 39), or can disregulate cellular genes near the integration site leading to tumorigenesis (21). Thus, lineage-specific regulation of expression will be beneficial for many gene therapy applications to avoid vector silencing or toxicities associated with unregulated transgene expression. Lineage-specific vectors will also be a useful tools for the study of lung biology, to track and identify stem cell progeny, monitor the differentiative potential of stem cells, and/or deliver transgenes at specific times or to specific lineages.
In this study we evaluated the lineage expression profile of lentiviral vectors with a series of respiratory epithelial specific regulatory elements. In cell line experiments, the SPC-promoted lentivector produced AECII-specific expression while the CC10-promoted lentivector had the highest level of expression in Clara cell lines. In contrast, the JSRV-promoted lentivector demonstrated a high level of transgene expression in many different cell types. These results are in contrast to the studies evaluating the JSRV promoter in plasmid and retroviral constructs, where expression is regulated to the AECII and Clara cell lineages. The reason for these differences in expression profile from the JSRV promoter among the different gene transfer vectors is unclear. However, we have also observed a similar phenomenon with the CD11b promoter, which produced regulated expression from plasmid vectors, transgenic mice (40, 41), and integrated retro-proviruses (42), but was disregulated and expressed in most cell types from a lentiprovirus (43, and C. Lutzko, unpublished data). It is possible that the remaining elements in the lentiviral backbone can affect the local chromatin structure to increase the recruitment of the transcriptional machinery (44). Another possibility is that the remaining transcription factor binding sites in the minimal LTR can cooperate with the JSRV promoter to enhance the transgene expression from integrated lentiproviruses in cell types in which the wild-type HIV is normally active. In support of this hypothesis, the highest level of expression was observed in Jurkat, a T-lymphoid cell line in which the wild-type HIV-1 promoter is active. However, further studies are needed to clarify the mechanism of the disregulation with the JSRV promoter in the lentiviral backbone.
Since the gene expression profile of cell lines does not exactly mirror normal primary cells, we also evaluated transgene expression from the CC10 and SPC lentivectors in respiratory and nonrespiratory tissues after intratracheal and intravenous delivery to murine recipients. Although there are several envelopes that provide higher levels of gene transfer to nasal or airway epithelium such as the baculovirus GP64 (20, 45) or filoviral envelopes (16, 17, 46), we chose to use VSV-G–pseudotyped lentiviral supernatant for the in vivo studies because it would enable transduction and expression analysis of a wide variety of cell types, due to its broad cellular tropism. This study would therefore provide data on the lineage restriction of transgene expression from our lentivectors. After administration of the CCL-CC10-EGFP lentivirus in vivo, transgene expression was exclusively observed in the lung, and not the heart, liver, spleen, or muscle. Surprisingly, characterization of the cell lineages expressing the EGFP transgene in the lung demonstrated that both Clara cells and AECII were EGFP positive. While the CC10 promoter is commonly used to direct transgene expression to Clara cells, other transgenic mouse studies have also observed transgene expression within the alveolus (29), although these authors were not able to identify the lineages expressing the transgene due to the low resolution of the methodology available at the time. Thus, our data indicate that incorporation of the CC10 promoter into lentiviral vectors results in respiratory-specific expression, which may be useful for the study of respiratory biology, or the regulated delivery of therapeutic genes to the airways and lungs.
Analysis of the transgene expression profile from the CCL-SPC-EGFP vector demonstrated that expression was restricted to AECII, as EGFP was not detected in nonrespiratory cell lines in vitro, nor the spleen, heart, or liver after intravenous delivery. While hematopoietic cells positive for EGFP protein were observed in the alveoli of recipient mice at early time points, they were undetectable by 6 weeks, and may have been the result of phagocytosis of EGFP protein in the viral preparation.
While the methods of intravenous and intratracheal in vivo delivery used in this study do not result in gene transfer to all tissues, they do provide data on the proviral expression analysis in a variety of primary tissue types, without the time and expense of generating transgenic mice. Although the mice used for intratracheal supernatant delivery studies were immune deficient, it is likely that these results will be similar to immune-competent mouse strains such as C57B6J, as there are several studies demonstrating long-term expression after in vivo delivery of lentivectors containing EGFP (47, 48) or the closely related EYFP (20).
Lentiviral vectors are easily modified to express genes of interest and readily produced with high titers suitable for in vivo administration. While the overall level of gene transfer to the airways and lungs was low in this study, the level was sufficient to carefully evaluate the lineages expressing the EGFP transgene. Several recent studies have described efficient delivery of other lentiviral vectors to the lungs of animals by altering the viral envelope (16–18), or increasing the viscosity of the supernatant by resuspending the virus in 1% methylcellulose (49) or LPC (19) to reduce the rate of clearance by the airways. Coupling the lineage-regulated vectors described here, with efficient delivery methods for the cell type of interest, will facilitate the development of safe and efficient gene therapies for respiratory disease, by producing lineage-regulated transgene expression. These vectors will also be of interest to respiratory biologists, as they will enable the regulated delivery of genes to the alveolar and airway epithelium, which can easily be modified to express a gene of interest specifically in the airway in Clara cells or the alveolus in AECII. Further, these vectors can also be used as a tool to mark and track the progeny of stem cells that have differentiated into specific respiratory lineages in vitro or in vivo. In summary, lentiviral vectors with regulated, alveolar and airway-specific expression profiles will be useful for the development of safe gene therapies, and the study of lung biology.
Acknowledgments
The authors thank Drs. Donald B. Kohn, Gay Crooks, and Paula Cannon for thoughtful discussions and reviewing the manuscript. They thank Dr. Jeffrey Whitsett for the SPC and CC10 promoters and the MLE-15 cell line, Dr. Hung Fan for the JSRV promoter, Dr. Donald B. Kohn for the MND promoter, and Dr. Franco De Mayo for Mtcc1-2 cells.
This work was supported by grants from the Department of Health and Human Services (HL072211), the Cystic Fibrosis Foundation, the Webb Foundation, and The Saban Research Institute at Childrens Hospital Los Angeles.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0276OC on June 15, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Klink D, Schindelhauer D, Laner A, Tucker T, Bebok Z, Schwiebert EM, Boyd AC, Scholte BJ. Gene delivery systems–gene therapy vectors for cystic fibrosis. J Cyst Fibros 2004;3:203–212. [DOI] [PubMed] [Google Scholar]
- 2.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 1999;274:10219–10226. [DOI] [PubMed] [Google Scholar]
- 3.Ilan Y, Droguett G, Chowdhury NR, Li Y, Sengupta K, Thummala NR, Davidson A, Chowdhury JR, Horwitz MS. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA 1997;94:2587–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croyle MA, Chirmule N, Zhang Y, Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol 2001;75:4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flotte TR. Adeno-associated virus-based gene therapy for inherited disorders. Pediatr Res 2005;58:1143–1147. [DOI] [PubMed] [Google Scholar]
- 6.Malik P, McQuiston SA, Yu XJ, Pepper KA, Krall WJ, Podsakoff GM, Kurtzman GJ, Kohn DB. Recombinant adeno-associated virus mediates a high level of gene transfer but less efficient integration in the K562 human hematopoietic cell line. J Virol 1997;71:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Densmore CL. Advances in noninvasive pulmonary gene therapy. Curr Drug Deliv 2006;3:55–63. [DOI] [PubMed] [Google Scholar]
- 8.Ziady AG, Davis PB, Konstan MW. Non-viral gene transfer therapy for cystic fibrosis. Expert Opin Biol Ther 2003;3:449–458. [DOI] [PubMed] [Google Scholar]
- 9.Sinn PL, Sauter SL, McCray PB Jr. Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors–design, biosafety, and production. Gene Ther 2005;12:1089–1098. [DOI] [PubMed] [Google Scholar]
- 10.Naldini L, Blomer U, Gage FH, Trono D, Verma MM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 1996;93:11382–11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park F, Ohashi K, Kay MA. The effect of age on hepatic gene transfer with self-inactivating lentiviral vectors in vivo. Mol Ther 2003;8:314–323. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K, Park F, Kay MA. Role of hepatocyte direct hyperplasia in lentivirus-mediated liver transduction in vivo. Hum Gene Ther 2002;13:653–663. [DOI] [PubMed] [Google Scholar]
- 13.Santoni de Sio FR, Cascio P, Zingale A, Gasparini M, Naldini L. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood 2006;107:4257–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 1998;72:9873–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borok Z, Harboe-Schmidt JE, Brody SL, You Y, Zhou B, Li X, Cannon PM, Kim KJ, Crandall ED, Kasahara N. Vesicular stomatitis virus G-pseudotyped lentivirus vectors mediate efficient apical transduction of polarized quiescent primary alveolar epithelial cells. J Virol 2001;75:11747–11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobinger GP, Weiner DJ, Yu QC, Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol 2001;19:225–230. [DOI] [PubMed] [Google Scholar]
- 17.Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, Sanders DA, McCray PB Jr. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J Virol 2003;77:5902–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvertown JD, Walia JS, Summerlee AJ, Medin JA. Functional expression of mouse relaxin and mouse relaxin-3 in the lung from an Ebola virus glycoprotein-pseudotyped lentivirus via tracheal delivery. Endocrinology 2006;147:3797–3808. [DOI] [PubMed]
- 19.Limberis M, Anson DS, Fuller M, Parsons DW. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum Gene Ther 2002;13:1961–1970. [DOI] [PubMed] [Google Scholar]
- 20.Kremer KL, Dunning KR, Parsons DW, and Anson DS. Gene delivery to airway epithelial cells in vivo: a direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. J Gene Med 2007;9:362–368. [DOI] [PubMed]
- 21.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003;302:415–419. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol 1998;72:8150–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutzko C, Senadheera D, Skelton D, Petersen D, Kohn DB. Lentivirus vectors incorporating the immunoglobulin heavy chain enhancer and matrix attachment regions provide position-independent expression in B lymphocytes. J Virol 2003;77:7341–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Indraccolo S, Minuzzo S, Roccaforte F, Zamarchi R, Habeler W, Stievano L, Tosello V, Klein D, Gunzburg WH, Basso G, et al. Effects of CD2 locus control region sequences on gene expression by retroviral and lentiviral vectors. Blood 2001;98:3607–3617. [DOI] [PubMed] [Google Scholar]
- 25.Kowolik CM, Hu J, Yee JK. Locus control region of the human CD2 gene in a lentivirus vector confers position-independent transgene expression. J Virol 2001;75:4641–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, Sadelain M. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 2000;406:82–86. [DOI] [PubMed] [Google Scholar]
- 27.Moreau-Gaudry F, Xia P, Jiang G, Perelman NP, Bauer G, Ellis J, Surinya KH, Mavilio F, Shen CK, Malik P. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood 2001;98:2664–2672. [DOI] [PubMed] [Google Scholar]
- 28.Korfhagen TR, Glasser SW, Wert SE, Bruno MD, Daugherty CC, McNeish JD, Stock JL, Potter SS, Whitsett JA. Cis-acting sequences from a human surfactant protein gene confer pulmonary-specific gene expression in transgenic mice. Proc Natl Acad Sci USA 1990;87:6122–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 1992;267:14703–14712. [PubMed] [Google Scholar]
- 30.Palmarini M, Datta S, Omid R, Murgia C, Fan H. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J Virol 2000;74:5776–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Challita PM, Skelton D, el-Khoueiry A, Yu XJ, Weinberg K, Kohn DB. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J Virol 1995;69:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbonaro DA, Jin X, Petersen D, Wang X, Dorey F, Kil KS, Aldrich M, Blackburn MR, Kellems RE, Kohn DB. In vivo transduction by intravenous injection of a lentiviral vector expressing human ADA into neonatal ADA gene knockout mice: a novel form of enzyme replacement therapy for ADA deficiency. Mol Ther 2006;13:1110–1120. [DOI] [PubMed] [Google Scholar]
- 33.Haas DL, Lutzko C, Logan AC, Cho GJ, Skelton D, Jin Yu X, Pepper KA, Kohn DB. The Moloney murine leukemia virus repressor binding site represses expression in murine and human hematopoietic stem cells. J Virol 2003;77:9439–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehrenbach H, Fehrenbach A, Pan T, Kasper M, Mason RJ. Keratinocyte growth factor-induced proliferation of rat airway epithelium is restricted to Clara cells in vivo. Eur Respir J 2002;20:1185–1197. [DOI] [PubMed] [Google Scholar]
- 35.Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 1994;93:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Slepushkin VA, Bodner M, Zabner J, van Es HH, Thomas P, Jolly DJ, Davidson BL, McCray PB Jr. Keratinocyte growth factor induced epithelial proliferation facilitates retroviral-mediated gene transfer to distal lung epithelia in vivo. J Gene Med 1999;1:22–30. [DOI] [PubMed] [Google Scholar]
- 37.Zsengeller ZK, Halbert C, Miller AD, Wert SE, Whitsett JA, Bachurski CJ. Keratinocyte growth factor stimulates transduction of the respiratory epithelium by retroviral vectors. Hum Gene Ther 1999;10:341–353. [DOI] [PubMed] [Google Scholar]
- 38.Bowtell DD, Johnson GR, Kelso A, Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med 1987;4:229–250. [PubMed] [Google Scholar]
- 39.Challita PM, Kohn DB. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci USA 1994;91:2567–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hickstein DD, Baker DM, Gollahon KA, Back AL. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc Natl Acad Sci USA 1992;89:2105–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dziennis S, Van Etten RA, Pahl HL, Morris DL, Rothstein TL, Blosch CM, Perlmutter RM, Tenen DG. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood 1995;85:319–329. [PubMed] [Google Scholar]
- 42.Bauer TR Jr, Osborne WR, Kwok WW, Hickstein DD. Expression from leukocyte integrin promoters in retroviral vectors. Hum Gene Ther 1994;5:709–716. [DOI] [PubMed] [Google Scholar]
- 43.Lutzko C, Yu X, Kohn DB. Consistent, lineage specific, myeloid expression from the CD11b promoter in retroviral gene transfer vectors carrying the scaffold attachment or insulator elements. Mol Ther 2001;3:S149. [Google Scholar]
- 44.Logan AC, Haas DL, Kafri T, Kohn DB. Integrated self-inactivating lentiviral vectors produce full-length genomic transcripts competent for encapsidation and integration. J Virol 2004;78:8421–8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinn PL, Burnight ER, Hickey MA, Blissard GW, McCray PB Jr. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J Virol 2005;79:12818–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina MF, Kobinger GP, Rux J, Gasmi M, Looney DJ, Bates P, Wilson JM. Lentiviral vectors pseudotyped with minimal filovirus envelopes increased gene transfer in murine lung. Mol Ther 2003;8:777–789. [DOI] [PubMed] [Google Scholar]
- 47.Pan D, Gunther R, Duan W, Wendell S, Kaemmerer W, Kafri T, Verma IM, Whitley CB. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther 2002;6:19–29. [DOI] [PubMed] [Google Scholar]
- 48.Worsham DN, Schuesler T, von Kalle C, Pan D. In vivo gene transfer into adult stem cells in unconditioned mice by in situ delivery of a lentiviral vector. Mol Ther 2006;14:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinn PL, Penisten AK, Burnight ER, Hickey MA, Williams G, McCoy DM, Mallampalli RK, McCray PB. Gene transfer to respiratory epithelia with lentivirus pseudotyped with Jaagsiekte sheep retrovirus envelope glycoprotein. Hum Gene Ther 2005;16:479–488. [DOI] [PubMed] [Google Scholar]