Abstract
Transforming growth factor (TGF)-β1 activity has been shown to increase vascular endothelial barrier permeability, which is believed to precede several pathologic conditions, including pulmonary edema and vessel inflammation. In endothelial monolayers, TGF-β1 increases permeability, and a number of studies have demonstrated the alteration of cell–cell contacts by TGF-β1. We hypothesized that focal adhesion complexes also likely contribute to alterations in endothelial permeability. We examined early signal transduction events associated with rapid changes in monolayer permeability and the focal adhesion complex of bovine pulmonary artery endothelial cells. Western blotting revealed rapid tyrosine phosphorylation of focal adhesion kinase (FAK) and Src kinase in response to TGF-β1; inhibition of both of these kinases using pp2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), ameliorates TGF-β1–induced monolayer permeability. Activation of FAK/Src requires activation of the epidermal growth factor receptor downstream of the TGF-β receptors, and is blocked by the epidermal growth factor receptor inhibitor AG1478. Immunohistochemistry showed that actin and the focal adhesion proteins paxillin, vinculin, and hydrogen peroxide–inducible clone-5 (Hic-5) are rearranged in response to TGF-β1; these proteins are released from focal adhesion complexes. Rearrangement of paxillin and vinculin by TGF-β1 is not blocked by the FAK/Src inhibitor, pp2, or by SB431542 inhibition of the TGF-β type I receptor, anaplastic lymphoma kinase 5; however, pp1 (4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), which inhibits both type I and type II TGF-β receptors, does block paxillin and vinculin rearrangement. Hic-5 protein rearrangement requires FAK/Src activity. Together, these results suggest that TGF-β1–induced monolayer permeability involves focal adhesion and cytoskeletal rearrangement through both FAK/Src-dependent and -independent pathways.
Keywords: focal adhesion complex, hydrogen peroxide–inducible clone 5, nuclear translocation, paxillin, vinculin
CLINICAL RELEVANCE
Pulmonary edema is a critical factor in acute respiratory distress syndrome. Transforming growth factor (TGF)-β1 is involved in this activity, and we have investigated early signal transduction of TGF-β1, which is involved in alterations of the endothelial cell monolayer.
Transforming growth factor (TGF)-β1, a multifunctional growth factor/cytokine, can activate numerous physiologic and pathologic cellular processes, such as cell migration, stimulation or inhibition of cell growth, tissue injury response, modulation of immune cell activity, and extracellular matrix modification (1–5). Paradoxically, although TGF-β1 is important for normal repair processes in several tissues, increased expression of TGF-β1 has a detrimental effect in the lung (5, 6). In pathologic conditions, TGF-β1 has been implicated in increased permeability of the endothelial barrier associated with both pulmonary edema and vascular remodeling. In patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) increased levels of TGF-β1 protein, detected in the bronchoalveolar lavage fluid, correlates with pulmonary edema and increased pulmonary vessel permeability (7–11). In animal models of ALI/ARDS, TGF-β1 protein is increased in the bronchoalveolar lavage fluid several days after administration (12), and pulmonary edema could be attenuated by either a pharmacologic inhibitor of TGF-β1 or in mice lacking the integrin αvβ6, which activates latent TGF-β1 (13, 14). TGF-β1 is also believed to be involved in the loss of pulmonary artery barrier function, leading to vascular remodeling in pulmonary hypertension; in this case, decreased endothelial barrier function allows cytokines and inflammatory cells infiltration of the intimal layer of the vessels, leading to smooth muscle cell hypertrophy and hyperplasia (15).
In vitro studies have shown that TGF-β1 induces an alteration in vascular endothelial cell shape from cobblestone to a pleomorphic morphology within 24 hours (16), and TGF-β1 reduces vascular endothelial monolayer barrier integrity that is detectable within 3 hours of treatment (17). The change in endothelial barrier permeability is concurrent with myosin light-chain phosphorylation, rearrangement of the myosin and actin cytoskeletal proteins, and disassembly of the adherens junctions (17, 18).
Members of the TGF-β superfamily signal through complexes of heteromeric serine/threonine kinase transmembrane receptors, which have been classified as type I or type II receptors based on amino acid sequences and functional properties (3, 6). Extensive research has shown that the biological responses to TGF-β1 are cell type specific, and that the signaling differences occur, at least in part, due to differential expression of TGF-β receptor subtypes (19, 20). Endothelial cells of the lung express the TGF-β type II receptor (TβR-II) and two type I receptors, the activin receptor-like kinase 5 (ALK5), which is broadly expressed, and ALK1, which is expressed solely in the endothelium (20). Signal transduction leading to loss of endothelial barrier function has been shown to include TGF-β1 activation of p38 mitogen-activated protein kinase (MAPK), and the activation of RhoA and Rho-kinase (17, 18, 21).
Recently, experiments have shown that focal adhesion kinase (FAK) is also important for the regulation of endothelial barrier function (22). We hypothesized that TGF-β1–induced membrane permeability may also involve alterations in the focal adhesion complex. Here, we have examined early TGF-β1 signal transduction leading to the activation of FAK and src kinase, and rearrangement of the focal adhesion proteins, paxillin, vinculin, and hydrogen peroxide–inducible clone (Hic)-5. Our results suggest that FAK/Src activation requires the epidermal growth factor receptor (EGFR) downstream of TGF-β receptor activation, and, although Src and FAK may regulate subcellular localization of some members of the focal adhesion complex (e.g., Hic-5), other members (e.g., paxillin and vinculin) are regulated through other pathways downstream of the TGF-β receptors.
MATERIALS AND METHODS
Reagents
TGF-β1 protein (101-B1) was purchased from R&D Systems (Minneapolis, MN). FBS (100–106) was from Gemini Bio-Products (Woodland, CA). RPMI 1640 medium, fungizone, and Dulbecco's PBS were purchased from Invitrogen (Carlsbad, CA). The FAK/Src kinase inhibitor, pp2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), and actinomycin D were purchased from Calbiochem (La Jolla, CA). The TGF-β type I receptor kinase inhibitors, SB431542 or SB505124, were purchased from Sigma Aldrich (St. Louis, MO). The EGFR inhibitor, AG1478, was purchased from Calbiochem (San Diego, CA); pp1 (4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) inhibitor was purchased from Tocris (Elliswille, MO). Antibodies were purchased from the following companies: anti-p42/p44 MAPK, anti-paxillin and anti-Smad2/3 were from Upstate Biotech (Charlottesville, VA); EGFR activated form (clone 74), vinculin, and Hic-5 antibodies were purchased from BD Transduction Laboratories (San José, CA); phospho-p42/p44 MAPK antibody was purchased from Signal Transduction Laboratories (Beverly MA); antibodies for extracellular signal–regulated kinase 1, β-actin, and influenza hemaglutinin (HA, Y-11) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488–labeled secondary antibodies directed against rabbit or mouse primary antibodies, and rhodamine-labeled phalloidin were purchased from Molecular Probes (Eugene, OR).
Cell Culture
Bovine pulmonary artery endothelial cells (BPAEC) purchased from Cell Applications, Inc. (San Diego, CA). Passage 2–8 cells were used for all experiments and were cultured in RPMI 1640 with 10% FBS, 1% penicillin/streptomycin, and 0.5% fungizone. Cells were grown in 5% CO2 at 37°C in a humidified atmosphere in a culture incubator. Human embryonic kidney 293 cells, purchased from American Type Culture Collection (Manassas, VA), were grown in Dulbecco's modified Eagle's medium with 10% FBS, 1% penicillin/streptomycin, and 0.5% fungizone, in 5% CO2 at 37°C in a humidified atmosphere in a culture incubator.
Endothelial Barrier Permeability
BPAEC were seeded on transparent filter insert (Falcon; BD Biosciences, Bedford, MA) preincubated in a six-well plate with warm RPMI/10% FBS. Once the cells grew to confluence (6–7 days), cells were switched to low serum (RPMI/0.01% FBS) for 16 hours. The endothelial barrier function was measured using MilliCell-ERS (Millipore, Billerica, MA). The electrical resistance of each cell monolayer was measured at several time points before and after different treatments by immersing one tip of the electrode in the well and the other in the insert. The level of total resistance of the electrode is subtracted as a control for all electrical resistance experiments. The resistance of the filter alone is approximately 98–100 mOhms. Experiments were performed at least three times (each with n = 3 monolayers). Data are presented as means (+ SD). Statistical differences were determined by analysis of variance followed by Bonferroni's post hoc testing for multiple comparisons between two sample means (P < 0.05 was considered statistically significant).
Cell Lysate and Immunoprecipitation
Cell lysates for Western blotting were prepared as previously described (23). Briefly, cells were growth-arrested in medium containing 0.1% FBS. The cells grown on a 35-mm dish were preincubated with inhibitors for 30 minutes before treatment with TGF-β1. Cells were washed two times with ice-cold PBS and then scraped in 100 μl buffer containing 50 mM Hepes solution (pH 7.4) containing 1% (vol/vol) Triton X-100, 4 mM EDTA, 1 mM NaF, 0.1 mM Na3VO4, 1 mM Na4P2O7, 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. After the cells were transferred to the tubes, they were placed on ice for 15 minutes and briefly vortexed. The insoluble material was removed by centrifugation (14,000 × g, 10 minutes, 4°C), and the supernatant was used for analysis.
For immunoprecipitation, equal concentrations of protein (at least 300 μg) of cell lysates were immunoprecipitated with the 1/1,000 dilution of primary antibody and 1/100 (vol/vol) GammaBind Plus beads (10 μl/1 ml; Amersham Biosciences, Piscataway, NJ). After incubation overnight on a rotator at 4°C, the beads were precipitated by microcentrifugation at 10,000 × g for 10 minutes at 4°C and washed twice with fresh lysis buffer containing protease and phosphatase inhibitors. The final wash buffer was removed and beads were boiled in 25 μl fresh Laemmli buffer for 5 minutes. The beads were vortexed and finally precipitated for 10 minutes at 14,000 × g.
Nuclear Extracts
Cells were washed in ice-cold PBS and scraped into a lysis buffer containing 10 mM Hepes (pH 7.8), 10 mM KCl, 2 mM MgCl2, 4 mM EDTA, 0.1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 95 mM NaF, 2.7 mM Na3VO4, and 10 mM Na4P2O7; lysates were incubated for 15 minutes at 4°C. Nonidet P-40 was then added at a final concentration of 0.6%. The lysates were then mixed vigorously and centrifuged at 4°C for 5 minutes. Pelleted nuclei were resuspended in 50 mM Hepes (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 0.1 mM PMSF, and 10% (vol/vol) glycerol, then mixed for 30 minutes to 2 hours at 4°C, centrifuged, and the supernatant harvested.
Western Blots
Cell lysates (10 μg of protein) were electrophoresed through 10% SDS-PAGE and electroblotted onto a membrane. Blots were blocked in 5% BSA in Tris-buffered saline (TBS)/0.1% Tween 20 (TTBS) for 1 hour at ambient temperature before incubating overnight with 1:1,000 dilution of primary antibody in TTBS/0.5% BSA at 4°C. Blots were washed three times with TTBS for 10 minutes, for a total of 30 minutes. Secondary antibodies were diluted 1:1,000 in TTBS for 1 hour; blots were washed in TBS for at least 2 hours before exposing to the film. ECL (Amersham, Piscataway, NJ) was applied according to the manufacturer's instructions before film exposure.
Immunohistochemistry
Cells were grown in 35-mm dishes onto glass coverslips for 8–16 hours. Cells were grown to 80% confluence before treatment. Cells were then washed once with 1 ml warm PBS for 3 minutes before fixing for 5 minutes in 1 ml formalin (4% formaldehyde in PBS). Cells were then gently washed thrice with 1 ml room temperature PBS for 3 minutes. Cells were permeabilized for 5 minutes with triton buffer (0.1% triton, 50 mM PIPES, pH 7.0, 90 mM HEPES, pH 7.0, 0.5 mM MgCl2, 75 mM KCl, 0.5 mM EGTA) and again washed three times in PBS. For actin cytoskeletal labeling, rhodamine-labeled phalloidin (1:50 in 1% BSA in PBS) was added drop-wise onto the center of the cover slip and incubated for 1 hour at ambient temperature. For immunodetection of focal adhesion proteins, primary antibodies were diluted 1:100 in 2% BSA in PBS. Antibody solutions were added drop-wise to the coverslip and incubated for 30 minutes at ambient temperature. Secondary antibodies were diluted 1:100 in 2% BSA in PBS and incubated for 30 minutes at ambient temperature. Finally, the stained cells were washed three times with 1 ml PBS at ambient temperature for 3 minutes each. Cover slips were mounted in 9:1 glycerol PBS. Fluorescent proteins were visualized on an Olympus FV500 series confocal laser scanning microscope using ×40 magnification (Olympus, Center Valley, PA).
Neutral Comet Assay
The neutral comet assay was used to measure double-stranded DNA breaks as an indication of apoptosis as previously described (24). Cells were treated with apoptotic stimuli, washed in PBS, pH 7.4, embedded in 1% agarose, and placed on a comet slide (Trevigen, Gaithersburg, MD). Cells were then placed in lysis solution (2.5 M NaCl, 1% Na-lauryl sarcosinate, 100 mM EDTA, 10 mM Tris base, 0.01% Triton X-100) for 30 minutes. The nuclei were subsequently electrophoresed for 20 minutes at 1 V/cm in 1× Tris/borate/EDTA buffer (TBE; 5× TBE stock is 250 mM Tris, 250 mM Boric acid, 5 mM EDTA), fixed in ethanol, followed by staining with Sybr Green (Molecular Probes, Eugene, OR) and visualized with an Olympus FV500 series confocal laser scanning microscope using 40× magnification at 478 nm excitation and 507 nm emission wavelengths. Between 100 and 150 comets were scored per experiment and assigned into type A, B, or C categories, based on their tail moments. Type C comets were defined as apoptotic cells as described by Krown and colleagues (25).
RESULTS
TGF-β1 Activates Src and FAK, and Inhibition of These Kinases Ameliorates TGF-β1–Induced Permeability of an Endothelial Monolayer
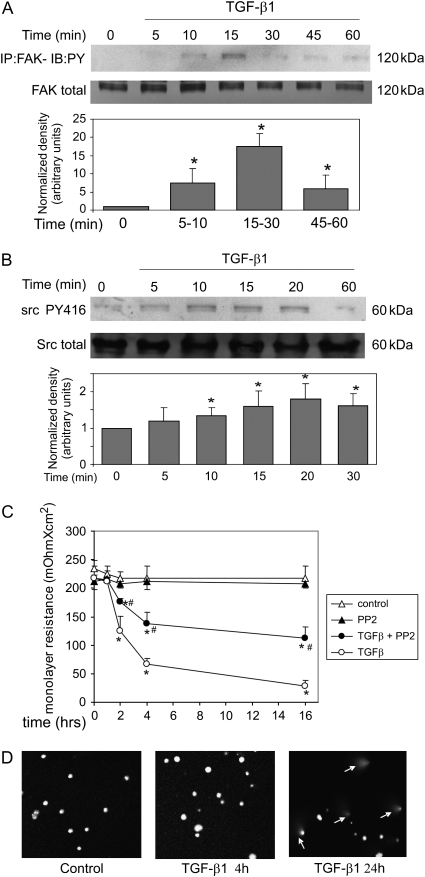
We hypothesized that focal adhesion complexes and cellular adhesion to the substratum may be involved in endothelial barrier function (22). A study of TGF-β1 signal transduction showed that FAK is activated downstream of integrin upregulation within approximately 6 hours in fibroblasts (26). The kinetics of TGF-β1–induced phosphorylation of FAK and Src is shown in Figure 1A. Phosphorylation occurred within 10 minutes after 1 ng/ml TGF-β1 treatment, and was transient. Interestingly, although Src is not phosphorylated in fibroblasts, we found phosphorylation of src in endothelial cells with a time course similar to that of FAK (Figure 1B). Our experiments also showed that total cellular levels of FAK and Src were not altered by treatment with TGF-β1 (middle panels, Figures 1A and 1B, respectively).
Figure 1.
Transforming growth factor (TGF)-β1–induced membrane permeability is ameliorated by focal adhesion kinase (FAK)/Src inhibition. (A and B) Bovine pulmonary artery endothelial cells (BPAEC) were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated with TGF-β1 (1 ng/ml) for the indicated times. (A) Equal protein concentrations of cell lysates were immunoprecipitated with anti-FAK and GammaBind beads. Immunoprecipitates were used for Western blots with antiphosphotyrosine. The blot was then stripped and probed for total FAK protein (middle panel). Experiments were repeated at least three times. Densitometry shows normalized increase in tyrosine phosphorylation (lower panel). *Statistically significant difference from corresponding control mean (n = 3). (B) Equal protein concentrations of whole-cell lysates were directly analyzed in Western blots using the phosphotyrosine 418 Src-specific antibody. The blot was then stripped and probed for total Src protein (middle panel). Experiments were repeated at least three times. Densitometry shows normalized increase in tyrosine phosphorylation (lower panel). *Statistically significant difference from corresponding control mean (n = 3). Representative data are shown for all blots. (C) BPAEC were grown to confluence on transparent filter insert. Cells were placed in low serum (0.01% FBS) overnight and then treated with TGF-β1 (1 ng/ml) with or without pretreatment with 10 μM pp2. Control cells were treated with vehicle (DMSO) or treated with 10 μM pp2 alone. Membrane permeability was measured at the indicated times, as described in Materials and Methods. Open triangles, control; closed triangles, pp2; closed circles, TGF-β + pp2; open circles, TGF-β. *Statistically significant difference from corresponding control mean; #statistically significant difference from corresponding TGF-β1 mean; P < 0.05. (D) Neutral comet assay. BPAEC were grown to 80 to 90% confluence, placed in low serum (0.01% FBS) overnight, and treated with 1 ng/ml TGF-β1. At the indicated times, cells were harvested and embedded in paraffin. Cells were lysed and DNA was electrophoresed in Tris/borate/EDTA (TBE) buffer. Rounded nuclei indicate the intact DNA; comet-like tails indicate apoptotic DNA fragmentation.
We next investigated the role of FAK/Src in TGF-β1–induced increase in endothelial monolayer permeability. Treatment of a confluent monolayer of PAEC with TGF-β1 reduced the integrity of the endothelial barrier, detectable within 2 hours (Figure 1C). This is in agreement with the findings by others using both macromolecular diffusion and electrical impedance techniques showing that TGF-β1 increases permeability of an endothelial monolayer (17). Pretreatment of cells with pp2, a FAK/Src inhibitor, ameliorated TGF-β1–induced permeability of the endothelial barrier. Treatment with pp2 alone had no detectable effect on the endothelial barrier permeability. In some cases, TGF-β1 has been shown to cause decreased viability of endothelial cells. We therefore investigated the effect of TGF-β1 on apoptosis in the PAEC during this short time period using the neutral comet assay (Figure 1D). The 4-hour time point showed no apoptosis, although some apoptosis was observed at 24 hours. Therefore, the increase in endothelial barrier permeability at early time points did not appear to be related to TGF-β1–induced apoptosis.
ALK5 Receptor Inhibition Does Not Block TGF-β1–Induced FAK Activation, but EGFR Transactivation Is Required
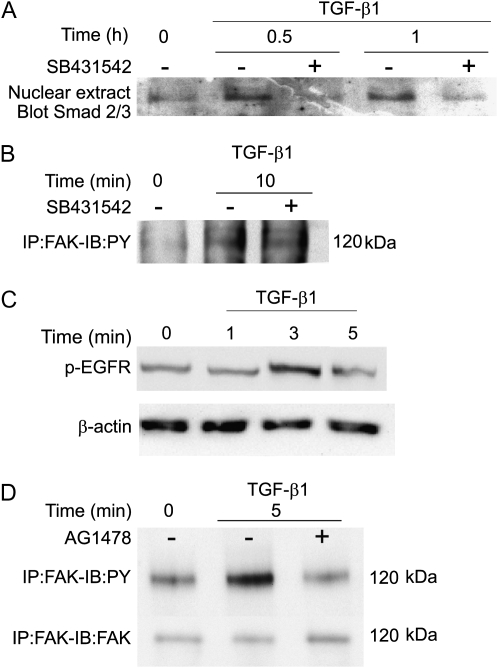
Endothelial cells express the TβR-II and two type I receptors, ALK1 and ALK5 (20). These two type I receptors appear to have different signaling targets in endothelial cells (20); however, the kinase activity of ALK5 was found to be required for ALK1-induced signal transduction, such as Smad1/5 phosphorylation (27). In agreement with findings by others (3), inhibition of the ALK5 receptor kinase by the specific inhibitor, SB431542, blocked TGF-β1–induced nuclear translocation of Smads 2 and 3 in PAEC (Figure 2A). Unexpectedly, however, phosphorylation of FAK was not affected by inhibition of ALK5 (Figure 2B); total levels of FAK were unchanged during this time course (data not shown).
Figure 2.
FAK activation does not require anaplastic lymphoma kinase (Alk) 5 but does require epidermal growth factor receptor (EGFR) transactivation. PAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without pretreatment with 10 μM SB431542. (A) Nuclear extracts were prepared, and equal amounts of protein from each condition were used for Western blots for Smad 2/3 protein. (B) Equal amounts of protein from whole-cell lysates were immunoprecipitated using anti-FAK antibody and GammaBind beads. Proteins were eluted from the beads and used for Western blotting with antiphosphotyrosine antibody. (C) Cells were treated for the indicated times with TGF-β1 (1 ng/ml). Equal protein concentrations of whole-cell lysates were analyzed in Western blots using the phosphotyrosine EGFR-specific antibody. The blot was stripped and probed for β-actin as a loading control. (D) Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without 20-minute pretreatment with 10 μM AG1478. Equal amounts of protein from whole-cell lysates were immunoprecipitated using anti-FAK antibody and GammaBind beads. Proteins were eluted from the beads and used for Western blotting with anti-phosphotyrosine antibody (upper panel); the blot was stripped and probed for total FAK protein as a loading control (lower panel). Representative data are shown for all blots.
Recent results by Uchiyama-Tanaka and colleagues showed that, in mesangial cells, TGF-β1 activates FAK through the rapid (∼ 2 minute) transactivation of the EGFR (28). Because of the similarity of the FAK activation time course in mesangial cell with our findings in PAEC, we investigated whether the EGFR was also involved in this cell type. Western blot analysis showed that TGF-β1 treatment led to the phosphorylation of EGFR, detectable within 3 minutes (Figure 2C). Preincubation of cells with the specific EGFR inhibitor, AG1478, completely blocked TGF-β1–induced phosphorylation of FAK without altering total cellular concentrations of FAK protein (Figure 2D). These data suggest that transactivation of EGFR is required for FAK activation, and that this activation occurs independently of ALK5 activation.
FAK/Src Inhibition Reduces TGF-β1–Induced Increase in Tyrosine Phosphorylation but Does Not Block P42/P44 MAPK Activation
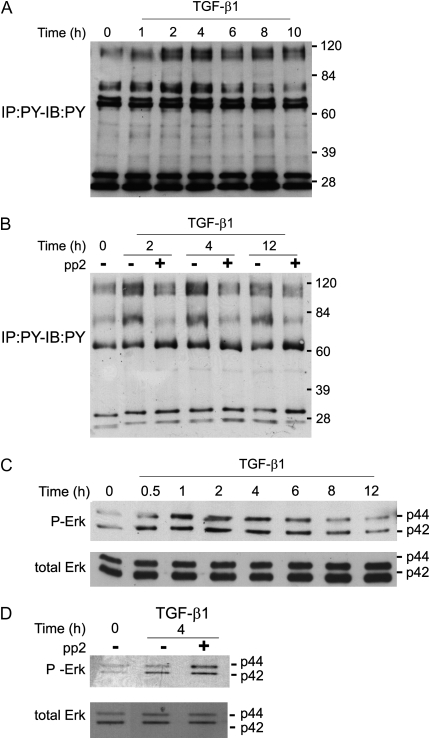
Our findings of FAK and Src activation by TGF-β1 in endothelial cells were in contrast with previous findings in fibroblasts, in which FAK activation is delayed (∼ 6 hours) and there is no activation of Src (26, 29). In fibroblasts, TGF-β1 signaling results in phosphorylation of a number of high–molecular weight proteins including FAK and additional proteins that have not yet been identified (30). Inhibition of FAK phosphorylation blocks some of the long-term effects of TGF-β1 in fibroblasts, including alterations associated with transdifferentiation to the myofibroblast phenotype (26). Examination of signaling by TGF-β1 in endothelial cells also showed the induction of tyrosine phosphorylation on several high molecular weight proteins (Figure 3A). A peak of tyrosine phosphorylation of a number of proteins (mostly > 60 kD) occurred between 2 and 4 hours after treatment; this is in contrast with findings in fibroblasts, where a peak in tyrosine phosphorylation of high–molecular weight proteins occurred at 6–12 hours (30). Inhibition of FAK/Src kinases in PAEC greatly reduced overall TGF-β1–induced tyrosine phosphorylation (Figure 3B), suggesting that FAK and/or Src are required for subsequent tyrosine phosphorylation.
Figure 3.
FAK/Src activation contributes to total TGF-β1–induced phosphotyrosine activation but not p42/p44 MAPK activation in PAEC. (A and B) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. (A) Cells were treated with TGF-β1 (1 ng/ml) for the indicated times; (B) cells were pretreated with or without 10 mM pp2 for 30 minutes. Equal protein concentrations of cell lysates were used for Western blots with antiphosphotyrosine. (C and D) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. (C) Cells were treated with TGF-β1 (1 ng/ml) for the indicated times; (D) cells were pretreated with or without 10 μM pp2 for 30 minutes. Equal protein concentrations of cell lysates were used for Western blots with anti–phospho-p42/p44 MAPK (upper panels of C and D) or total p42/p44 MAPK (lower panels of C and D). Representative data are shown.
As the identities of most of the phosphotyrosine proteins are not known, we further investigated the requirement of FAK/Src for TGF-β1 activation of MAPK in endothelial cells. In PAEC, TGF-β1 induced sustained activation of MAPK, which was detectable within 30 minutes of treatment (Figure 3C). Total cellular levels of MAPK were not changed. Interestingly, pp2 did not inhibit TGF-β1 activation of MAPK, suggesting that FAK/Src are not required for MAPK activation by TGF-β1 (Figure 3D).
TGF-β1 Induces Cytoskeletal and Focal Adhesion Complex Rearrangement
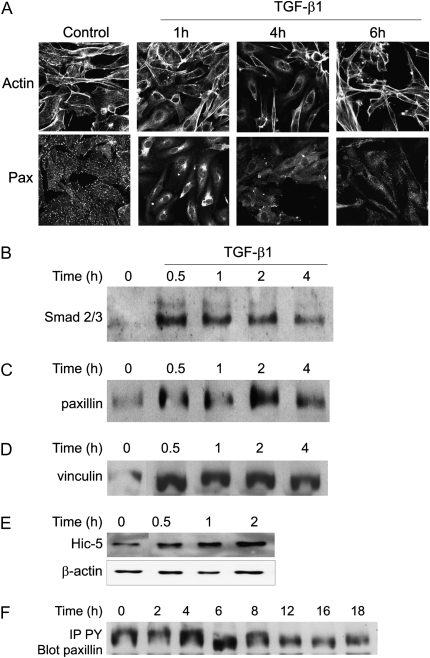
The phosphorylation states of FAK and/or Src are known to influence the assembly of proteins in the focal adhesion complex (31). Treatment of PAEC with TGF-β1 induced the rearrangement of peripheral actin, which could be detected by immunohistochemical staining within 1 hour of treatment, with a maximal effect at approximately 4 hours (Figure 4A, upper panels). Actin fibers withdrew from the periphery of the cell, and subsequently formed a network within the cell, away from the cell membrane. No reduction in total cellular actin was detected (data not shown). This is in agreement with findings by others in studies examining actin rearrangement in microvascular endothelial cells in response to factors that alter cellular motility or cell monolayer integrity (17, 21, 32).
Figure 4.
TGF-β1 induces reorganization of the actin cytoskeleton and proteins from the focal adhesion complex. (A) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated with TGF-β1 (1 ng/ml) for the indicated times. Cells were then fixed, permeabilized, and stained for the actin cytoskeleton (upper panels) or paxillin (Pax; lower panels). (B–E) BPAEC were grown to 80–90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated with TGF-β1 (1 ng/ml) for the indicated times. Nuclear extracts were harvested and equal amounts of protein were subjected to Western blots for: (B) Smad 2/3; (C) paxillin; (D) vinculin; and (E) hydrogen peroxide–inducible clone (Hic)-5 (this blot was also stripped and probed for β-actin as a loading control [lower panel]). (F) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated with TGF-β1 (1 ng/ml) for the indicated times. Equal amounts of protein from cell lysates were immunoprecipitated using anti-phosphotyrosine antibody and GammaBind beads. Immunoprecipitates were then used for Western blots for paxillin. Representative results are shown for all experiments.
Rearrangement of focal adhesion proteins paxillin, vinculin, and the paxillin-related protein, Hic-5, were observed with a similar time course as actin rearrangement. In untreated cells, paxillin was observed in discretely staining focal adhesions, as shown by immunohistochemical staining (Figure 4A, lower panels). Within 1 hour of treatment with TGF-β1, paxillin protein appeared to be removed from the focal adhesion complexes, and could be detected in a network within the cell. Similar findings were obtained for rearrangement of vinculin and Hic-5 (data not shown).
Previous findings by others have shown that paxillin and Hic-5 are translocated to the nucleus under certain conditions (33, 34). The TGF-β1–induced network of focal adhesion proteins appeared near the nucleus, and we tested the ability of the rearranged proteins to associate with the nucleus in a manner that would allow their copurification. Treatment of PAEC with TGF-β1 induced the nuclear translocation of Smad2/3 proteins, which are known to be involved in TGF-β1 signaling, within 30 minutes, and these proteins were detected in a Western blot of nuclear extract (Figure 4B). We were also able to detect the translocation of paxillin, vinculin, and Hic-5 within 30 minutes, and the association with the nucleus was maintained for up to 4 hours (respectively, Figures 4C–4E). We also observed an increase in paxillin phosphorylation as demonstrated by immunoprecipitation with anti-phosphotyrosine antibody followed by immunoblotting with anti-paxillin antibody (Figure 4F). The increased phosphorylation correlates with the time course of rearrangement of paxillin from the focal adhesion.
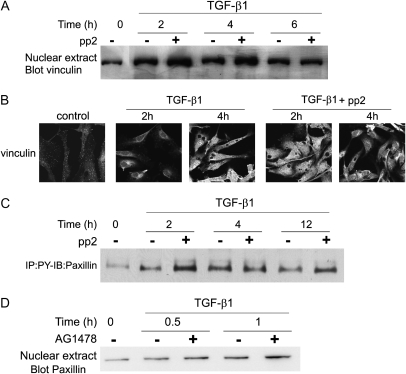
FAK/Src and EGFR Inhibition Do Not Prevent TGF-β1–Induced Rearrangement of Vinculin and Paxillin
Rearrangement of focal adhesion proteins has been shown in several systems to be controlled by FAK and/or Src activity (31). We investigated the role of these proteins in vinculin and paxillin rearrangement by inhibiting FAK and Src with pp2. Results show that TGF-β1–induced rearrangement of vinculin rearrangement was not inhibited by pretreatment with pp2. Immunohistochemical results showed that pretreatment of cells with pp2 did not prevent vinculin from being localized away from focal adhesions in response to TGF-β1 treatment (Figure 5A). Similar findings were obtained for paxillin rearrangement (data not shown). Western blots of nuclear extracts showed that vinculin was associated with the nucleus after TGF-β1 treatment and was not blocked by pretreatment with pp2 (Figure 5B). The pp2 inhibitor also failed to prevent TGF-β1–induced phosphorylation of paxillin (Figure 5C).
Figure 5.
TGF-β1–induced reorganization of the focal adhesion proteins does not require FAK/Src activation or EGFR transactivation. (A–C) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without pretreatment with 10 μM pp2. Cells were then used for the following series of experiments. (A) Nuclear extracts were prepared and equal amounts of protein from each sample were used for Western blots for vinculin. (B) Cells were fixed, permeabilized, and analyzed by immunohistochemistry using anti-vinculin antibody. (C) Equal amounts of protein from whole-cell lysates were immunoprecipitated with anti-phosphotyrosine antibody and GammaBind beads; proteins eluted from the beads were used for Western blots for paxillin protein. (D) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without 20-minute pretreatment with 10 μM AG1478. Nuclear extracts were prepared, and equal amounts of protein from each condition were used for Western blots for paxillin. Representative results are shown for all blots.
As shown in Figure 2C, the EGFR is rapidly phosphorylated in response to TGF-β1. We investigated whether focal adhesion protein rearrangement required this transactivation. The EGFR inhibitor, AG1478, also did not block rearrangement of paxillin as determined by Western blot of nuclear extracts (Figure 5D). This is in agreement with our finding that FAK/Src activation is not upstream of vinculin rearrangement.
ALK5 Is Not Specifically Required for Vinculin and Paxillin Rearrangement by TGF-β1
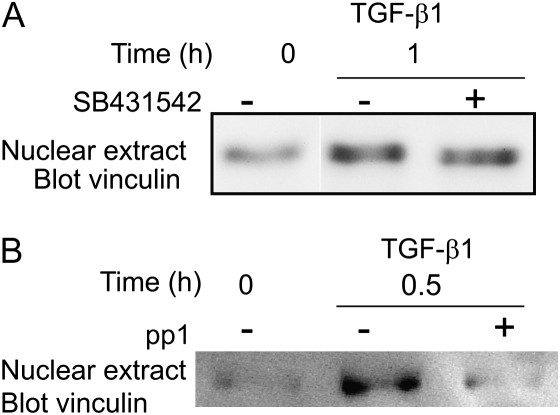
Endothelial cells of the lung express two type I TGF-β receptors: ALK5, which is broadly expressed, and ALK1, which is expressed solely in the endothelium (20). We wished to determine which of these receptors is required for focal adhesion protein reorganization. In Figure 2A, we demonstrate the ALK5-dependent activation of Smad2/3 in PAEC. However, TGF-β1–induced rearrangement of the focal adhesion protein, vinculin, was not blocked by ALK5 inhibitor, SB431542, as determined by Western blot analysis (Figure 6A). This indicates that, although ALK5 is clearly activated in PAEC and is required for Smad2/3 activation, it is not required for vinculin rearrangement.
Figure 6.
TGF-β1–induced reorganization of vinculin and paxillin is blocked by combined inhibition of TGF-β type I and type II receptors. PAEC were grown to 80 to 90% confluence and were placed in low serum (0.01% FBS) overnight. Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without pretreatment with 10 μM SB431542 or SB505124. (A) Nuclear extracts were prepared and equal amounts of protein were used for Western blots for vinculin. (B) Cells were treated for the indicated times with TGF-β1 (2 ng/ml) with or without 20-minute pretreatment with 10 μM pp1. Nuclear extracts were prepared, and equal amounts of protein from each condition were used for Western blots for vinculin.
We next attempted to specifically inhibit ALK1 using adenoviral expression of a dominant-negative mutant (gift of Dr. P. ten Dijke, The Netherlands Cancer Institute, Amsterdam, The Netherlands [35]). However, infection of PAEC with control adenovirus abrogated the cytoskeletal responses to TGF-β1, suggesting that this method of protein expression would not be suitable for our system. Recently, the pp1 inhibitor was shown to inhibit both type I and type II TGF-β receptors (36). We used this drug to determine whether simultaneous inhibition of the type I and type II TGF-β receptors could block vinculin rearrangement. Preincubation of cells with pp1 effectively blocked vinculin rearrangement, suggesting that signal transduction through the TGF-β receptors are required for this activity (Figure 6B).
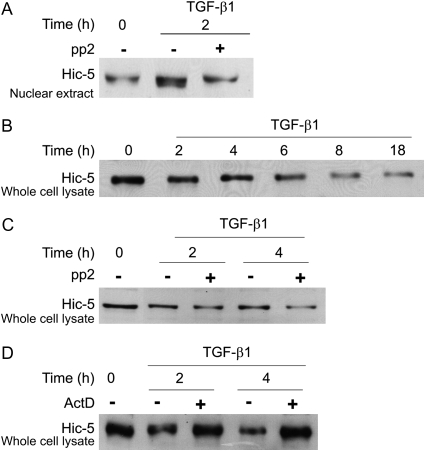
FAK/Src Inhibition Prevents TGF-β1–Induced Rearrangement of Hic-5
Rearrangement of focal adhesion proteins has been shown to occur simultaneously with activation of FAK and Src kinases. In several cases, activation of FAK or Src has been shown to occur upstream of alteration of the focal adhesions (31, 37, 38). Inhibition of FAK/Src kinases by pp2 blocked TGF-β1–induced nuclear association of Hic-5 at 2 hours as shown by a Western blot of nuclear extract (Figure 7A). Interestingly, we observed that TGF-β1 induced significant downregulation of total cellular Hic-5 protein in PAEC, observable within 2–4 hours in Western blots of whole-cell lysate (Figure 7B); this is in contrast with findings from other osteoblastic and fibroblast cell types, in which TGF-β1 induces Hic-5 expression (39, 40). Down-regulation of the total cellular Hic-5 protein was not inhibited by pp2, but could be blocked by actinomycin D (Figures 7C and 7D, respectively). These findings together suggest that rearrangement of Hic-5 by TGF-β1 requires FAK/Src activation; however, the TGF-β1–induced decrease in total cellular Hic-5 is FAK/Src-independent, but may be regulated through gene transcription.
Figure 7.
Hic-5 rearrangement by TGF-β1 requires FAK/Src activity, but Hic-5 downregulation requires new protein synthesis. (A) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without pretreatment with 10 μM pp2. Nuclear extracts were harvested and equal amounts of protein were subjected to Western blots for Hic-5. (B–D) BPAEC were grown to 80 to 90% confluence and placed in low serum (0.01% FBS) overnight. Cells were treated for the indicated times with TGF-β1 (1 ng/ml) with or without pretreatment with: (B) no inhibitors; (C) 10 μM pp2; or (D) 5 μg/ml actinomycin D. Whole-cell lysates were used in Western blots for Hic-5 protein.
DISCUSSION
The primary finding of this work is that TGF-β1 induces the rapid activation of FAK and src associated with the early loss of barrier function in an endothelial cell monolayer. TGF-β1 also induces the rearrangement of focal adhesion proteins, paxillin, vinculin, and Hic-5, as well as actin cytoskeleton reorganization with a similar time course. Our experiments using the pp2 inhibitor indicate that redistribution of paxillin and vinculin is independent of FAK/Src activation, but FAK/Src activity is required for Hic-5 rearrangement. We have also determined that the TGF-β1 transactivation of EGFR is necessary for FAK/Src activation, but not for vinculin rearrangement. Finally, the TGF-β1 type 1 receptor, ALK5, is not required for this activity, but combined inhibition of the type I and type II TGF-β receptors by pp1 does block focal adhesion rearrangement. Several laboratories have shown that the two type I receptors, ALK1 and ALK5, have different targets in endothelial cells (e.g., ALK1 is upstream of Smads 5 and 8, whereas ALK5 is upstream of Smad3 activation). Unfortunately, our experiments using adenoviral expression of dominant-negative ALK1 could not be interpreted due to the inhibition of the vinculin/paxillin rearrangement by the adenoviral infection itself. Therefore, our results cannot determine whether there is redundancy in the ALK1, ALK5, and/or the type II receptor signaling, or whether the signaling to vinculin/paxillin occurs via ALK1 and/or the type II receptor. We can conclude that FAK/Src activations are not required for this event.
Our findings indicate that the focal adhesion proteins, paxillin and vinculin, are transiently redistributed away from focal adhesions to internal cellular locations, and that these proteins can copurify with the nucleus. This redistribution of paxillin and/or vinculin has been demonstrated to occur in other cell types in response to factors that induce cellular motility, such as in hepatocyte growth factor treatment of smooth muscle cells (41) and in platelet-derived growth factor treatment of NIH 3T3 cells (42). In both of these examples, focal adhesion rearrangement is believed to play a role in cellular motility, but the function of relocalization of focal adhesion proteins to a nucleus-associated region is not currently understood. We hypothesize that the rearrangement of focal adhesion proteins may function to shuttle transcription factors to the perinuclear region. The paxillin-related protein, Hic-5, may provide an example of this function. Hic-5 is localized to the focal adhesion complex in resting cells and is translocated into the nucleus in response to a variety of signaling events where it regulates the activity of transcription factors, such as c-fos and the specificity protein 1 (SP1) transcription factor (34, 43, 44).
Two studies have shown that TGF-β1–induced endothelial barrier dysfunction involves activation of p38. The findings show that p38 is phosphorylated within 30 minutes to 1 hour after TGF-β1 stimulation of BPAEC (18), and that this activation requires Smad2 protein (45). It is likely that the activation of p38 is independent of and not required for FAK/src phosphorylation as the time course of p38 activation is far downstream our time course of FAK/Src phoshorylation, and we have shown that FAK/Src activation is independent of ALK5 which activates Smad2. We have performed preliminary studies that show that p38 inhibition using SB203580 does not block TGF-β1–induced vinculin rearrangement (data not shown).
We have additionally identified differential effects of TGF-β1 on Hic-5 expression in endothelial cells, where Hic-5 is downregulated in a mechanism that requires new transcription. Other researchers have found that Hic-5 levels increase in response to TGF-β1 treatment of osteoblasts, rat embryo fibroblast-52 cells (REF52), and mesenchymally transdifferentiated epithelial cells (40, 46, 47). Recent findings by several laboratories have identified Hic-5 as a steroid receptor coactivator (48) as well as a coregulator of Smad3 and Sp1 (44, 49, 50). Our finding, that TGF-β1 downregulates Hic-5 protein in PAEC, suggests that the transcriptional regulatory activity of Hic-5 is also differentially regulated in PAEC in response to TGF-β1. This is in contrast with the upregulation of Hic-5 as seen in REF52 or other mesenchymal cell types. Hic-5 protein has also been shown to be required for endothelial cellular migration; either phosphorylation or knockdown of Hic-5 blocked motility (51, 52). Thus, TGF-β1–induced degradation of Hic-5, in combination with alterations in paxillin and vinculin subcellular localization, could potentially play a role in the concerted regulation of cell motility and transcriptional regulation related to cell migration. Current work in our laboratory is directed toward further understanding of possible cell type differences in the function of Hic-5 in modulation of transcription factor activity in primary PAEC.
The differential effects of TGF-β1 on different cell types can be demonstrated by comparison of our current findings in primary endothelial cells with findings from mesenchymal cell types. TGF-β1 signaling in cultured endothelial cells can induce loss of barrier function, growth arrest, and, in some cases, apoptosis (20, 21). This is in contrast with TGF-β1 signaling in primary lung fibroblasts, which leads to proliferation and hypertrophy (26). Our signal transduction findings in pulmonary endothelial cells reveal significant differences from signal transduction events previously shown to occur in primary fibroblasts in response to TGF-β1 (26). Activation of FAK and Src by TGF-β1 in endothelial cells occurs within 10–20 minutes, whereas studies of TGF-β1 signaling in fibroblasts have shown that FAK activation is not detected until 8–12 hours (26), and src activation has not been reported. We also found peak activation of total cellular tyrosine phosphoproteins by TGF-β1 in endothelial cells at 2 to 4 hours. In contrast, fibroblasts do not show maximal tyrosine phosphorylation until 10 to 16 hours after TGF-β1 treatment (30). The activation time course of p42/p44 MAPK by TGF-β1 in endothelial cells also differs from that in fibroblasts. Whereas endothelial cells have significant p42/p44 MAPK activation within 1 hour of treatment with TGF-β1, fibroblasts do not display detectable activity until 6 to 16 hours. In fibroblasts, the delayed activation of FAK occurs after synthesis of extracellular matrix proteins and autocrine activation of integrins (26). Delayed activation of p42/p44 MAPK also requires expression of basic fibroblast growth factor and autocrine activation of its receptor (53). Our results here demonstrate that early activation of FAK/Src requires transactivation of EGFR; the mechanism of p42/p44 MAPK activation is still under investigation in PAEC.
Much of the cell type–specific signaling differences of TGF-β1 have been attributed to differential expression of the TGF-β receptor types (20). Our findings indicate that TGF-β1 regulates cellular attachment to the extracellular matrix in addition to regulating cell–cell adhesion, as shown by others. The combination of these events is likely important for the overall alterations in the endothelial barrier during ARDS, leading to infiltration of inflammatory cells as well as increased pulmonary edema (9, 54). The use of mice lacking the α5β6 integrin, which activates latent TGF-β1, helped to identify TGF-β1 as a critical effector of pulmonary edema after ALI (14, 54). Given the importance of TGF-β1 in both normal and pathologic processes, it will be important to continue to learn how the differential expression of the TGF-β receptor subtypes affects activation of downstream signaling pathways.
Acknowledgments
The authors thank Autumn J. Griffin for technical support. The opinions expressed in this document are those of the authors and do not reflect the views of the Uniformed Services University of the Health Sciences, the U.S. Department of Defense, or the U.S. federal government.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL79320 (U.K.) and R01HL073929, and by Uniformed Services University of the Health Sciences grant CO75LE (R.M.D.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0439OC on June 21, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-β superfamily and Runx proteins. Oncogene 2004;23:4232–4237. [DOI] [PubMed] [Google Scholar]
- 2.de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-β signaling in cancer. J Natl Cancer Inst 2000;92:1388–1402. [DOI] [PubMed] [Google Scholar]
- 3.Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-β superfamily. Cytokine Growth Factor Rev 1996;7:327–339. [DOI] [PubMed] [Google Scholar]
- 4.Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol Biotechnol 2004;28:147–166. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lazaro JF, Thieringer F, Luth S, Czochra P, Meyer E, Renteria IB, Galle PR, Lohse AW, Herkel J, Kanzler S. Hepatic over-expression of TGF-β1 promotes LPS-induced inflammatory cytokine secretion by liver cells and endotoxemic shock. Immunol Lett 2005;101:217–222. [DOI] [PubMed] [Google Scholar]
- 6.de Caestecker M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev 2004;15:1–11. [DOI] [PubMed] [Google Scholar]
- 7.Budinger GR, Chandel NS, Donnelly HK, Eisenbart J, Oberoi M, Jain M. Active transforming growth factor-β1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Med 2005;31:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groeneveld AB, Polderman KH. Acute lung injury, overhydration or both? Crit Care 2005;9:136–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesnutt AN, Kheradmand F, Folkesson HG, Alberts M, Matthay MA. Soluble transforming growth factor-α is present in the pulmonary edema fluid of patients with acute lung injury. Chest 1997;111:652–656. [DOI] [PubMed] [Google Scholar]
- 10.Luchtel DL, Embree L, Guest R, Albert RK. Extra-alveolar veins are contiguous with, and leak fluid into, periarterial cuffs in rabbit lungs. J Appl Physiol 1991;71:1606–1613. [DOI] [PubMed] [Google Scholar]
- 11.Lamm WJ, Luchtel D, Albert RK. Sites of leakage in three models of acute lung injury. J Appl Physiol 1988;64:1079–1083. [DOI] [PubMed] [Google Scholar]
- 12.Yi ES, Salgado M, Williams S, Kim SJ, Masliah E, Yin S, Ulich TR. Keratinocyte growth factor decreases pulmonary edema, transforming growth factor-β and platelet-derived growth factor-BB expression, and alveolar type II cell loss in bleomycin-induced lung injury. Inflammation 1998;22:315–325. [DOI] [PubMed] [Google Scholar]
- 13.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-β: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med 2003;31:S258–S264. [DOI] [PubMed] [Google Scholar]
- 14.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. TGF-β is a critical mediator of acute lung injury. J Clin Invest 2001;107:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109:159–165. [DOI] [PubMed] [Google Scholar]
- 16.Coomber BL. Cytoskeleton in TFG-β- and bFGF-modulated endothelial monolayer repair. Exp Cell Res 1991;194:42–47. [DOI] [PubMed] [Google Scholar]
- 17.Hurst VI, Goldberg PL, Minnear FL, Heimark RL, Vincent PA. Rearrangement of adherens junctions by transforming growth factor-β1: role of contraction. Am J Physiol 1999;276:L582–L595. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg PL, MacNaughton DE, Clements RT, Minnear FL, Vincent PA. p38 MAPK activation by TGF-β1 increases MLC phosphorylation and endothelial monolayer permeability. Am J Physiol Lung Cell Mol Physiol 2002;282:L146–L154. [DOI] [PubMed] [Google Scholar]
- 19.Merwin JR, Newman W, Beall LD, Tucker A, Madri J. Vascular cells respond differentially to transforming growth factors β 1 and β 2 in vitro. Am J Pathol 1991;138:37–51. [PMC free article] [PubMed] [Google Scholar]
- 20.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-β receptor function in the endothelium. Cardiovasc Res 2005;65:599–608. [DOI] [PubMed] [Google Scholar]
- 21.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-β–induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 2005;288:L294–L306. [DOI] [PubMed] [Google Scholar]
- 22.Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol 2002;539:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day RM, Cioce V, Breckenridge D, Castagnino P, Bottaro DP. Differential signaling by alternative HGF isoforms through c-Met: activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene 1999;18:3399–3406. [DOI] [PubMed] [Google Scholar]
- 24.Kitta K, Day RM, Ikeda T, Suzuki YJ. Hepatocyte growth factor protects cardiac myocytes against oxidative stress–induced apoptosis. Free Radic Biol Med 2001;31:902–910. [DOI] [PubMed] [Google Scholar]
- 25.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CG, Quintana PJE, Sabbadini RA. Tumor necrosis factor α–induced apoptosis in cardiac myocytes: involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest 1996;98:2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 2003;278:12384–12389. [DOI] [PubMed] [Google Scholar]
- 27.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol Cell 2003;12:817–828. [DOI] [PubMed] [Google Scholar]
- 28.Uchiyama-Tanaka Y, Matsubara H, Mori Y, Kosaki A, Kishimoto N, Amano K, Higashiyama S, Iwasaka T. Involvement of HB-EGF and EGF receptor transactivation in TGF-β–mediated fibronectin expression in mesangial cells. Kidney Int 2002;62:799–808. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda K, Kawata S, Tamura S, Matsuda Y, Inui Y, Igura T, Inoue S, Kudara T, Matsuzawa Y. Transforming growth factor-β1–induced degradation of activated Src tyrosine kinase in rat fibroblasts. Oncogene 1998;16:3349–3356. [DOI] [PubMed] [Google Scholar]
- 30.Thannickal VJ, Aldweib KD, Fanburg BL. Tyrosine phosphorylation regulates H2O2 production in lung fibroblasts stimulated by transforming growth factor β1. J Biol Chem 1998;273:23611–23615. [DOI] [PubMed] [Google Scholar]
- 31.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol 1997;9:187–192. [DOI] [PubMed] [Google Scholar]
- 32.Lomnytska M, Lukiyanchuk V, Hellman U, Souchelnytskyi S. Transforming growth factor-β1–regulated proteins in human endothelial cells identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2004;4:995–1006. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa M, Hiraoka Y, Aiso S. Nuclear translocation of Xenopus laevis paxillin. Biochem Biophys Res Commun 2003;304:676–683. [DOI] [PubMed] [Google Scholar]
- 34.Shibanuma M, Kim-Kaneyama JR, Ishino K, Sakamoto N, Hishiki T, Yamaguchi K, Mori K, Mashimo J, Nose K. Hic-5 communicates between focal adhesions and the nucleus through oxidant-sensitive nuclear export signal. Mol Biol Cell 2003;14:1158–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J 2004;23:4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda M, Shintani Y, Wheelock MJ, Johnson KR. Src activation is not necessary for transforming growth factor (TGF)-β–mediated epithelial to mesenchymal transitions (EMT) in mammary epithelial cells: PP1 directly inhibits TGF-β receptors I and II. J Biol Chem 2006;281:59–68. [DOI] [PubMed] [Google Scholar]
- 37.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 1999;71:435–478. [DOI] [PubMed] [Google Scholar]
- 38.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005;6:56–68. [DOI] [PubMed] [Google Scholar]
- 39.Untergasser G, Gander R, Lilg C, Lepperdinger G, Plas E, Berger P. Profiling molecular targets of TGF-β1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech Ageing Dev 2005;126:59–69. [DOI] [PubMed] [Google Scholar]
- 40.Shibanuma M, Mashimo J, Kuroki T, Nose K. Characterization of the TGF β 1–inducible Hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem 1994;269:26767–26774. [PubMed] [Google Scholar]
- 41.Ma H, Calderon TM, Kessel T, Ashton AW, Berman JW. Mechanisms of hepatocyte growth factor–mediated vascular smooth muscle cell migration. Circ Res 2003;93:1066–1073. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez C, Portela RA, Mellado M, Rodriguez-Frade JM, Collard J, Serrano A, Martinez AC, Avila J, Carrera AC. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J Cell Biol 2000;151:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghogomu SM, Vanvenrooy S, Ritthaler M, Wedlich D, Gradl D. Hic-5, a novel repressor of Lef/Tcf driven transcription. J Biol Chem 2005;281:1755–1764. [DOI] [PubMed] [Google Scholar]
- 44.Shibanuma M, Kim-Kaneyama JR, Sato S, Nose KA. LIM protein, Hic-5, functions as a potential coactivator for Sp1. J Cell Biochem 2004;91:633–645. [DOI] [PubMed] [Google Scholar]
- 45.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-β1–induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol 2006;101:375–384. [DOI] [PubMed] [Google Scholar]
- 46.Thomas SM, Hagel M, Turner CE. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J Cell Sci 1999;112:181–190. [DOI] [PubMed] [Google Scholar]
- 47.Tumbarello DA, Brown MC, Hetey SE, Turner CE. Regulation of paxillin family members during epithelial–mesenchymal transformation: a putative role for paxillin δ. J Cell Sci 2005;118:4849–4863. [DOI] [PubMed] [Google Scholar]
- 48.Saelzler MP, Spackman CC, Liu Y, Martinez LC, Harris JP, Abe MK. ERK8 down-regulates transactivation of the glucocorticoid receptor through Hic-5. J Biol Chem 2006;281:16821–16832. [DOI] [PubMed] [Google Scholar]
- 49.Kim-Kaneyama J, Shibanuma M, Nose K. Transcriptional activation of the c-fos gene by a LIM protein, Hic-5. Biochem Biophys Res Commun 2002;299:360–365. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Song K, Sponseller TL, Danielpour D. Novel function of androgen receptor–associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J Biol Chem 2005;280:5154–5162. [DOI] [PubMed] [Google Scholar]
- 51.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol 2005;171:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hetey SE, Lalonde DP, Turner CE. Tyrosine-phosphorylated Hic-5 inhibits epidermal growth factor–induced lamellipodia formation. Exp Cell Res 2005;311:147–156. [DOI] [PubMed] [Google Scholar]
- 53.Finlay GA, Thannickal VJ, Fanburg BL, Paulson KE. Transforming growth factor-β 1–induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem 2000;275:27650–27656. [DOI] [PubMed] [Google Scholar]
- 54.Sheppard D. Transforming growth factor β: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 2006;3:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]