Abstract
Asthmatic airway remodeling is characterized by goblet cell hyperplasia, angiogenesis, smooth muscle hypertrophy, and subepithelial fibrosis. This study evaluated whether acquired changes in fibroblast phenotype could contribute to this remodeling. Airway and parenchymal fibroblasts from control or chronically ovalbumin (OVA)-sensitized and challenged “asthmatic” mice were assessed for several functions related to repair and remodeling ± exogenous transforming growth factor (TGF)-β. All OVA-challenged mouse fibroblasts demonstrated augmented gel contraction (P < 0.05) and chemotaxis (P < 0.05); increased TGF-β1 (P < 0.05), fibronectin (P < 0.05), and vascular endothelial growth factor (P < 0.05) release; and expressed more α-smooth muscle actin (P < 0.05). TGF-β1 stimulated both control and asthmatic fibroblasts, which retained all differences from control fibroblasts for all features(P < 0.05, all comparisons). Parenchymal fibroblasts proliferated more rapidly (P < 0.05), while airway fibroblasts proliferated similarly compared with control fibroblasts (P = 0.25). Thus, in this animal model, OVA-challenged mouse fibroblasts acquire a distinct phenotype that differs from control fibroblasts. The augmented profibrotic activity and mediator release of asthmatic fibroblasts could contribute to airway remodeling in asthma.
Keywords: remodeling, fibroblast, phenotype, mouse model
CLINICAL RELEVANCE
Lung fibroblasts cultured from ovalbumin-sensitized mice manifest persistent differences in vitro in phenotypes related to repair and remodeling. This suggests that acquired alterations of fibroblast repair phenotypes can contribute to the pathogenesis of asthma.
Asthma is a disorder characterized by chronic inflammation of the airways, airway hyperresponsiveness, and changes in airway architecture, sometimes termed remodeling (1–3). The cells responsible for the maintenance of lung structure are the parenchymal cells of the lung, including epithelial cells, mesenchymal cells, and endothelial cells. Recent studies have suggested that the function of epithelial cells (4), smooth muscle cells (5), and fibroblasts (6, 7) cultured from the lungs of individuals with asthma differs from the function of cells similarly cultured from individuals without asthma. These functional differences, particularly as they relate to repair and remodeling, could contribute to airway structural alterations.
The current study was designed to determine if the milieu present in the asthmatic airway could lead to the accumulation of an acquired population of phenotypically distinct mesenchymal cells. To accomplish this, the chronic ovalbumin (OVA)-sensitized and -challenged mouse model of allergic asthma was used (8, 9). In this model, mice develop both airway hyperresponsiveness and, after some weeks, manifest airway remodeling that resembles key features of chronic asthma (10, 11). Fibroblasts were cultured from both the large airways and lung parenchyma of control and OVA-sensitized and -challenged animals, and functional phenotypes related to repair and remodeling were assessed. The results of this study support the concept that phenotypically distinct populations of fibroblasts with augmented repair functions can accumulate in the airways in response to chronic OVA challenge, providing a mechanism to account for persistent lung structural remodeling.
MATERIALS AND METHODS
Materials
Native type I collagen (rat tail tendon collagen [RTTC]) was extracted from rat tail tendons as described (12). Commercially available reagents were obtained as follows: anti–transforming growth factor (TGF)-β1 monoclonal antibody (clone: 9016.2), TGF-β1, biotinylated anti–TGF-β1, anti-mouse vascular endothelial growth factor (VEGF) monoclonal antibody, biotinylated anti-mouse VEGF antibody, and mouse VEGF were from R&D Systems (Minneapolis, MN); 3,3′,5,5′-Tetramethyl benzidine (TMB) was from Sigma (St. Louis, MO). Peroxidase-conjugated anti-rabbit IgG antibody was from Rockland (Gilbertsville, PA). Murine fibronectin, Dulbecco's Modified Eagle's Medium (DMEM), and fetal calf serum (FCS) were from Invitrogen Life Technologies (Grand Island, NY).
Murine Model of Allergic Asthma
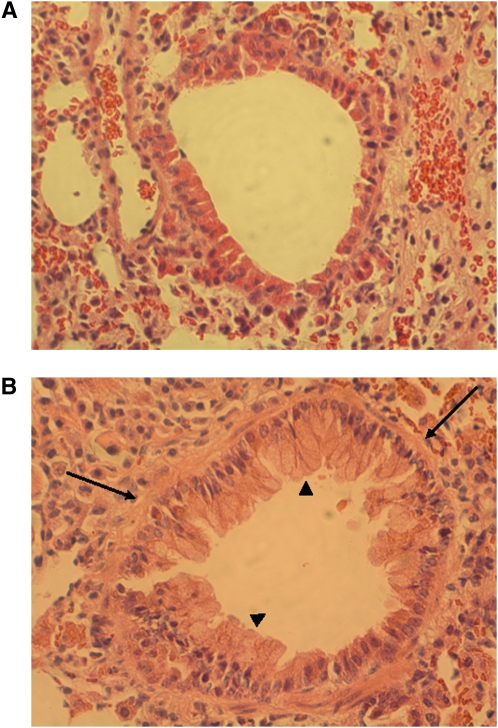
Female Balb/c mice were sensitized to OVA or treated with saline as controls as described (8–10). (Additional details are available in the online supplement.) The mice were killed on Day 39. As expected, OVA exposure resulted in readily detectable airway remodeling (Figure 1).
Figure 1.
Histology of sections of airways from control (A) or ovalbumin (OVA)-challenged mice (B). Sections were stained with hematoxylin and eosin. Magnification: ×200. Thickened basement membrane (arrows) and goblet cell hyperplasia (arrowheads) were seen around the airway wall in OVA-challenged mice.
Fibroblast Culture
After anesthesia, mice were killed. The thorax was opened and then the trachea and lungs were removed. Airways, including trachea and mainstem bronchi, were dissected and separated from parenchymal tissues, which included airways smaller than mainstem bronchi. These tissues were then minced and incubated with DMEM containing 15% FCS, as pilot studies demonstrated more reliable initiation of cell outgrowths than with lower serum concentrations. Cultures were refed three times weekly. All cultures were evaluated by immunohistochemistry to assess vimentin and keratin (13) and all stained positively with vimentin and failed to stain with keratin (see Figure E1 in the online supplement). Selected cultures were evaluated by transmission electron microscopy, and the morphology of the cells was consistent with that of fibroblasts/myofibroblasts (Figure E1). For simplicity, the term “fibroblast” will be used throughout the text. Fibroblasts were passaged with trypsin/EDTA (0.05% trypsin, 0.53 mM EDTA-4Na; GIBCO, Grand Island, NY). Fibroblasts between the third and sixth passages were used for assays.
Collagen Gel Contraction Assay
Collagen gels were prepared as described previously (14) so that cells were present at 5 × 105 cells/ ml, and the final concentration of collagen was 0.75 mg/ml. (Additional details are available in the online supplement.) The gels were incubated at 37°C in a 5% CO2 atmosphere for 3 days. Gel contraction was quantified using an Optomax V image analyzer (Optomax, Burlington, MA) daily. Data were expressed as percentage of the original gel size.
Chemotaxis Assay
Cell migration was assessed using the Boyden blindwell chamber (Neuroprobe Inc., Gaithersburg, MD) as previously described (15). (Additional details are available in the online supplement.) Cells were allowed to migrate at 37°C in a 5% CO2 atmosphere for 6 hours using fibronectin (20 μg/ml) as the chemoattractant. Chemotaxis was assessed by counting the number of cells in five high-power fields (5 HPF). Wells with SF-DMEM were used as negative controls, and those with chemoattractant alone were used as positive controls.
Fibroblast Proliferation Assay
Fibroblasts (1 × 105 cells per each well) were plated in 12-well plates in DMEM containing 15% FCS on Day 0. On Days 1, 3, 5, and 7, cells were trypsinized and cell number was counted by Coulter counter (Beckman Coulter Inc., Fullerton, CA).
Measurement of TGF-β1, Fibronectin, and VEGF by Enzyme-Linked Immunosorbent Assay
TGF-β1 (16), fibronectin (17), and VEGF (16) concentrations in the media of fibroblasts maintained in collagen gel culture were determined by enzyme-linked immunosorbent assay. (Additional details are available in the online supplement.)
Statistical Analysis
Data from the functional bioassays are expressed as mean ± SEM. The Mann-Whitney's U-test was used for single comparisons. Probability values of less than 0.05 were considered significant.
RESULTS
Histologic examination of lung sections from control and OVA-challenged mice were performed. As expected, in the airways of allergic OVA-challenged mice, thickened basement membrane and goblet cell hyperplasia were observed, whereas no pathologic change was found in the airways of control mice (Figure 1). While formal morphometric studies were not conducted, the fibrotic tissue remodeling appeared to have increased numbers of fibroblast-like cells.
Fibroblast-Mediated Collagen Gel Contraction
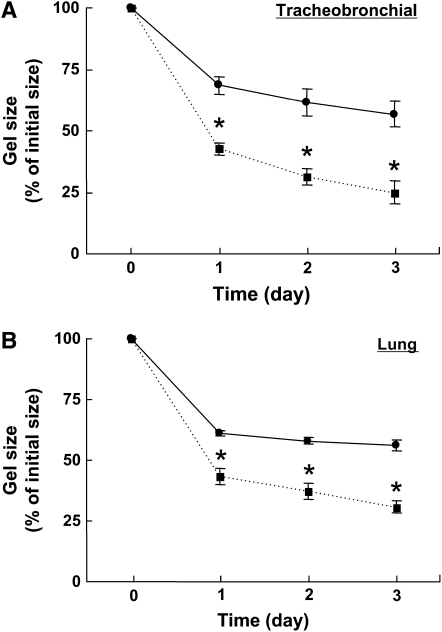
Collagen gel contraction mediated by OVA-challenged mouse tracheobronchial fibroblasts was significantly augmented compared with fibroblasts from control animals at all time points assessed. At the end of the experimental period on Day 3, OVA-challenged mouse fibroblasts reduced the gel size to 25.0 ± 4.4% of its initial size, whereas control fibroblasts only reduced the gel to 56.7 ± 5.2% of its initial size (P < 0.05, Figure 2A). Similarly, collagen gel contraction mediated by OVA-challenged mouse lung fibroblasts was significantly augmented compared with control; on Day 3, gel size was 30.6 ± 2.5% versus 56.1 ± 2.4% of initial size (P < 0.05, Figure 2B).
Figure 2.
Collagen gel contraction mediated by fibroblasts from control or OVA-challenged mice. Fibroblasts from control (closed circles) or OVA-challenged (closed squares) mice were cast into collagen gels and maintained in floating culture. (A) Airway fibroblasts (control, n = 4; OVA-challenged, n = 4). (B) Lung fibroblasts (control, n = 4; OVA-challenged, n = 5). Gel size was measured daily. Vertical axes: gel size (% of initial size); horizontal axes: time (day). All values are mean ± SEM for four to five separate mice, each evaluated in triplicate. *P < 0.05 compared with the values of control.
Fibroblast Chemotaxis
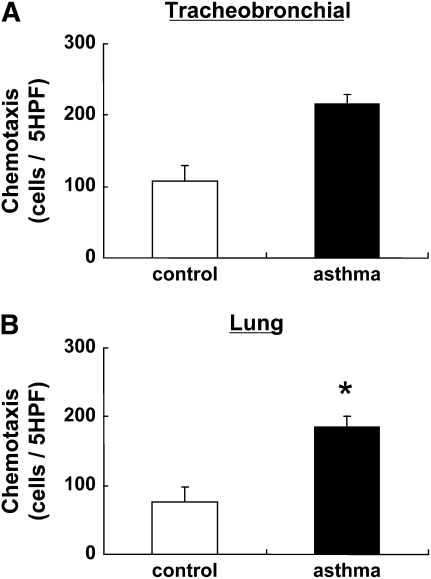
To evaluate chemotactic activity of control and OVA-challenged mouse fibroblasts, we assessed fibroblasts chemotaxis using fibronectin (20 μg/ml) as the chemoattractant. Chemotactic activity of OVA-challenged mouse tracheobronchial fibroblasts was significantly enhanced compared with control (215 ± 15 cells/5 HPF versus 109 ± 21 cells/5 HPF; P < 0.05, Figure 3A). Similarly, chemotactic activity of OVA-challenged mouse lung fibroblasts was significantly enhanced compared with control lung fibroblasts (185 ± 15 cells/5 HPF versus 76.6 ± 22 cells/5 HPF; P < 0.05, Figure 3B).
Figure 3.
Chemotactic activity toward fibronectin of fibroblasts from control or OVA-challenged mice. Fibroblasts from control (open bars) or OVA challenged (closed bars) mice were prepared in monolayer culture, harvested, and assayed for chemotaxis toward fibronectin (see Materials and Methods). (A) Airway fibroblasts (control, n = 4; OVA-challenged, n = 4). (B) Parenchymal fibroblasts (control, n = 4; OVA-challenged, n = 5). Vertical axes: chemotaxis (cells/5 high-power fields [HPF]). All values are mean ± SEM for four to five separate mice, each evaluated in triplicate. *P < 0.05 compared with the values of control.
Fibroblast Proliferation
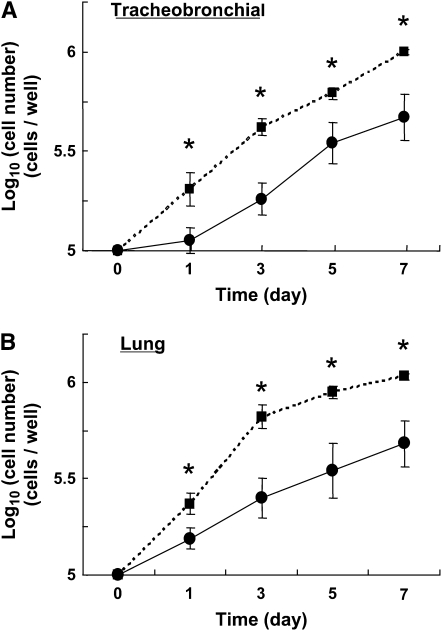
To investigate proliferation of fibroblasts from control and OVA-challenged mice, we assessed the increase in fibroblast number in cells cultured in DMEM containing 15% FCS. The initial cell counts were greater for OVA-challenged mouse tracheobronchial and lung fibroblasts than for controls, suggesting increased attachment and/or survival of the OVA-challenged mouse cells (Figure 4). Proliferation was gauged by the rate of replication. For tracheobronchial fibroblasts, the proliferation rate, as assessed by the initial doubling time, was similar for OVA-challenged mouse and control fibroblasts (3.40 ± 0.69 days versus 4.34 ± 0.30 days, P = 0.25). In contrast, the doubling time for OVA-challenged mouse lung fibroblasts was significantly less than that of control lung fibroblasts (2.03 ± 0.26 days versus 5.57 ± 1.5 days, P < 0.05), indicating that OVA-challenged mouse lung fibroblasts proliferate faster than control.
Figure 4.
Proliferation of fibroblasts from fibroblasts from control or OVA-challenged mice. Control (closed circles) or OVA-challenged (closed squares) mouse fibroblasts (1 × 105 cells/well) were plated with 15% fetal calf serum containing media in 12-well plates. Cells were then counted on Days 1, 3, 5, and 7. (A) Airway fibroblasts; (B) parenchymal fibroblasts. Vertical axes: cell number (×104 cells/well); horizontal axes: time (day). All values are mean ± SEM for four to five separate mice, each evaluated in triplicate. *P < 0.05 compared with the values of control.
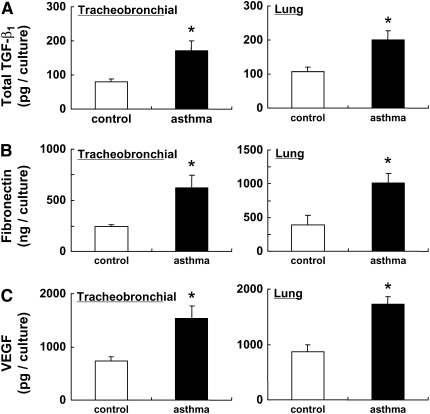
Because fibroblasts can release mediators that modulate repair and remodeling responses, including TGF-β1, fibronectin, and VEGF, the release of these mediators was assessed in fibroblasts cultured in three-dimensional collagen gels (Figure 5). The release of TGF-β (Figure 5A), fibronectin (Figure 5B), and VEGF (Figure 5C) were all increased in fibroblasts obtained from OVA-challenged mice compared to control mice. This was true for both tracheobronchial and lung fibroblasts (Figure 4).
Figure 5.
Release of transforming growth factor (TGF)-β1, fibronectin, and vascular endothelial growth factor (VEGF) from control and OVA-challenged mouse fibroblasts. Tracheobronchial fibroblast (left panels: control, n = 4; OVA-challenged, n = 4) or lung fibroblasts (right panels: control, n = 4; OVA-challenged, n = 5) were cast into three-dimensional (3D) collagen gels and maintained in floating culture in medium. After 3 days, media were harvested and assayed for TGF-β1, fibronectin, and VEGF by enzyme-linked immunosorbent assay. (A) Total TGF-β1 (pg/culture); (B) fibronectin (ng/culture); (C) VEGF (pg/culture). All values are mean ± SEM for four to five separate mice, each evaluated in duplicate experiments. *P < 0.05 compared with the values of control.
α-SMA Expression in Fibroblasts
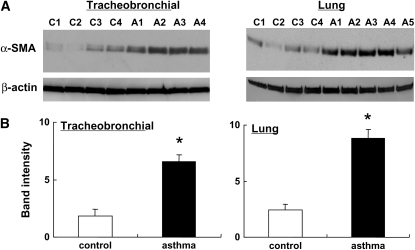
α-SMA is a marker of myofibroblasts and is believed to play a pivotal role in tissue remodeling. Therefore we assessed α-SMA expression in fibroblasts from control and OVA-challenged mice. OVA-challenged mouse tracheobronchial fibroblasts had significantly higher protein expression of α-SMA compared to control fibroblasts (P < 0.05, Figures 6A and 6B, left panel). A similar increase was observed in lung fibroblasts from OVA-challenged mice (P < 0.05, Figures 6A and 6B, right panel).
Figure 6.
α-smooth muscle actin (α-SMA) in fibroblasts from control or OVA-challenged mice in baseline condition. Tracheobronchial and control fibroblasts from control mice were cultured in monolayer, grown to 75% confluence, and immunoblotting for α-SMA was performed. (A) Immunoblots of tracheobronchial (left) and lung (right) fibroblasts. The Western blot is shown for four control mice (C1–C4) and for OVA-challenged mice (A1–A4, A5). (B) Relative intensity versus α-actin. All values are mean ± SEM. *P < 0.05 compared with the values of control.
Effect of TGF-β1 on Control and OVA-Challenged Mouse Fibroblasts
TGF-β1 can stimulate collagen gel contraction, chemotaxis and α-SMA expression, as well as fibronectin and VEGF production by fibroblasts, and can function as an autocrine or paracrine mediator. To determine if the increased activity of the OVA-challenged mouse fibroblasts was due to increased response to TGF-β, we evaluated the effect of exogenously administered TGF-β1 on control and OVA-challenged mouse fibroblasts. As expected, TGF-β1 significantly augmented contraction of control collagen gels, chemotaxis, production of fibronectin and of VEGF, and expression of α-SMA for both tracheobronchial and lung fibroblasts (Figures E3, E4, E5, and E6). Importantly, TGF-β1 also augmented OVA-challenged mouse tracheobronchial and lung fibroblasts, and the effects were significantly greater (P < 0.05 all comparisons) than for control cells for all parameters.
DISCUSSION
The present study evaluated functional phenotypes related to repair and remodeling in fibroblasts obtained from control mice and from OVA-sensitized and -challenged mice, a model of asthma that results in airway hyperresponsiveness and chronic airway remodeling. Compared with control fibroblasts, fibroblasts obtained from both the large airways and the lung parenchyma of the “asthmatic” mice demonstrated augmented chemotaxis toward fibronectin and contraction of three-dimensional collagen gels; produced more TGF-β1, fibronectin, and VEGF; and demonstrated increased production of α-SMA, a marker of myofibroblast differentiation. Lung parenchymal fibroblasts from OVA-challenged mice also proliferated more rapidly than fibroblasts from control mice, while tracheobronchial fibroblasts proliferated similarly. Exogenous TGF-β1 increased all the above measures in both control and OVA-challenged mouse fibroblasts, and all the differences compared with control and OVA-challenged mouse cells observed under “baseline” culture conditions persisted in the presence of exogenous TGF-β1. Taken together, the results of the current study demonstrate that chronic allergen exposure, which is associated with the development of airway remodeling, is also associated with the accumulation within the lung of populations of fibroblasts with a distinct phenotype characterized by augmented remodeling functions. Accumulation of these cells may be a key mechanism leading to the structural changes present in the allergic asthmatic airway.
Structural alterations of the airway wall, sometimes termed “airway remodeling,” are characteristic of the airways of patients with asthma and include epithelial cell metaplasia, smooth muscle hypertrophy, increased vascularity, and accumulations of extracellular matrix, including collagen (2, 3, 18). Thickening of the subepithelial basement membrane is a highly characteristic feature (19). While the physiologic consequences of the thickened basement membrane are undetermined, correlation with airway hyperresponsiveness has been reported, suggesting a link between altered structure and function (20). Basement membrane thickness also correlates with the number of airway myofibroblasts (19). As these are the cells believed to be responsible for the production and reorganization of extracellular matrix, alterations in parenchymal cell populations in the airway may be key in the pathogenesis of altered structure and function.
A number of mechanisms could contribute to the development of airway remodeling (2, 3, 18). In this context, airway parenchymal cells can respond to mediators present in the inflammatory milieu of the asthmatic airway. Both Th2 cytokines and TGF-β, for example, can have “profibrotic” effects on target mesenchymal cells, including the induction of α-SMA and other myofibroblast-like features in fibroblasts (21, 22). Thus, inflammatory mediators may contribute to the structural changes present in the airway. Anti-inflammatory therapy, however, has not been associated with a striking alteration in the structural changes present in the airway (23, 24). In addition, conventional anti-inflammatory therapy may not prevent the long-term functional consequences associated with asthma. While inflammatory mediators may contribute, mechanisms that could progress in the absence of inflammation would be consistent with these clinical observations.
Primary alteration in parenchymal cell behavior is an alternate mechanism that could lead to the structural changes present in asthma. In this context, functional alterations have been observed in epithelial cells (4), smooth muscle cells (5), and fibroblasts (6, 7) cultured from the airways of individuals with asthma. These functional differences persist during in vitro culture, suggesting these alterations are independent, or have become independent, of inflammatory cytokines. In the report of Lewis and colleagues, fibroblasts cultured from biopsies of individuals with severe asthma were found to express greater amounts of platelet-derive growth factor (PDGF) receptor beta and to produce more pro-collagen type I in response to PDGF BB (25). This suggests that repair and remodeling functions of asthmatic fibroblasts could differ from normal and could contribute to the structural alterations present in the asthmatic airway.
Several nonexclusive mechanisms could account for differences between epithelial and mesenchymal cells in asthma and controls. First, these differences may reflect an underlying genetic difference. In this regard, polymorphisms associated with the TGF-β gene have been associated with asthma (26, 27). In the presence of an underlying genetic alteration, the cells present in the asthmatic airway may be predisposed to undergo remodeling, and this would be reflected in the behavior of cells cultured from the asthmatic lung.
The results obtained in the present study, however, are consistent with an acquired difference. The current study used chronic OVA exposure in previously sensitized mice as a model of asthmatic airway remodeling. In this system, OVA sensitization and exposure results in airways inflammation associated with structural changes that resemble asthmatic airway remodeling (9, 28), including airway wall thickening, subepithelial fibrosis, and goblet cell hyperplasia (9, 28). Since the fibroblasts cultured from the lungs of both OVA-challenged and control mice are syngeneic, an underlying genetic difference cannot account for the phenotypic differences observed.
Several mechanisms could potentially lead to acquired phenotypic differences. The inflammatory milieu present in the airways could lead to the recruitment and expansion of populations of fibroblasts/myofibroblasts that are distinct from those present in tissues in control animals (29). Alternatively, the milieu present in the OVA-sensitized and -challenged animals could have induced differentiation of fibroblasts/myofibroblasts from precursor cell populations (30). These precursors could be local or derived from the circulation (30). These two mechanisms, recruitment and differentiation, could both be operative and may be synergistic.
The fibroblasts isolated from the murine lungs were cultured as populations. While the cells were defined as fibroblasts based on positive staining for vimentin and negative staining for keratin and were further characterized by ultrastructure, it is likely the cultured populations are heterogeneous. A shift in the distribution of subpopulations versus a change in the differentiated state of a single population cannot be determined from the current study. Indeed, the lack of markers specific for mesenchymal cell populations makes resolving this issue problematic. In addition, the studies characterizing fibroblasts were performed on cell populations after several passages in culture. Thus, any changes in the number of fibroblasts in the tissue would not be reflected in the current analyses.
The present study evaluated primary cultures of murine fibroblasts. These were evaluated between passages 3 and 6. Thus, the phenotypic differences observed are stable for at least this number of passages in vitro. Whether the changes observed represent “stable” differentiation that would persist for longer durations in culture is not addressed by the current study. In preliminary studies, murine lung fibroblasts have been found to proliferate inconsistently after passages 8 to 10, making more extended studies technically problematic. While the persistence of the altered differentiated state present in the OVA-challenged mouse lung fibroblasts cannot be further addressed, it is clear that fibroblasts maintained in vitro in the absence of an inflammatory milieu can manifest differences for at least several cell passages.
The present study was designed to assess phenotypic differences that might relate to the structural remodeling present in asthmatic airways. As such, fibroblast functions associated with repair were evaluated. It is of interest, therefore, that for all responses observed, the OVA-challenged mouse fibroblasts were more active than controls with the sole exception of the proliferation rate of tracheobronchial fibroblasts, which showed a nonsignificant trend toward increased proliferation. These results are consistent with a more exuberant response of asthmatic fibroblasts, which might be expected to lead to the fibrotic and hypervascular alterations present in the asthmatic lung (2, 3, 18). Interestingly, these differences were observed both for tracheobronchial fibroblasts cultured from the large airways and from lung parenchymal fibroblasts cultured from a mince of the distal lung.
A recent study has demonstrated functional heterogeneity of fibroblasts obtained from the proximal and distal lung (31). Kotaru and colleagues found that airway fibroblasts differed morphologically, in their expression of α-SMA and in their ability to produce type I collagen and eotaxin when compared with distal lung fibroblasts. The current study is consistent with this regional heterogeneity as distinct differences in gene expression were observed between tracheobronchial fibroblasts and fibroblasts cultured from the lung parenchyma (data not shown). It was not possible to dissect alveolar structures from small airways, nor was it possible to dissect airways from vascular structures. Thus, it is not possible to state with certainty the origin of the fibroblasts comprising the lung parenchymal population. Importantly, both populations of fibroblasts cultured from the lungs of OVA-challenged animals were distinctly different from control.
The current study focused on fibroblasts, which are believed to be cells that play a major role in the maintenance and remodeling of interstitial connective tissue (32). In this context, fibroblasts are believed to play a key role in maintaining and altering tissue structure. The ability of fibroblasts to migrate in response to chemotactic stimuli (33) and to proliferate in response to specific growth factors (34) is believed to control their accumulation at sites undergoing tissue repair. The ability of fibroblasts to produce and remodel extracellular matrix is thought to contribute to tissue structural changes (19). Remodeling of tissues likely involves fibroblast contractile activity. Fibroblasts can attach to fibrillar collagen through α2β1 integrin–dependent mechanisms (35). By exerting mechanical tension, fibroblasts can cause collagenous tissue matrices to contract. This is thought to play a key role in the contraction that characterizes normal scar formation as well as fibrotic tissues (35). Tissue contraction may account for narrowing of the small airways, which is a major determinant of fixed airflow limitation.
Fibroblasts can also produce a variety of regulatory molecules (16). The current study evaluated three: TGF-β, fibronectin, and VEGF. Each has been suggested to play a major role in modulating tissue repair and remodeling (16). The current study evaluated soluble culture media. Many molecules including TGF-β, VEGF, and fibronectin can bind to extracellular matrix. Thus, the measurements made in the soluble media likely reflect only part of the total produced by the cells. However, the data available clearly indicate a difference between the fibroblasts obtained from OVA-challenged and control animals. While increased numbers of fibroblast like cells may be present in tissues, it is unlikely that increased numbers of fibroblasts account for the increased mediator production observed in the current study. While time course experiments were not performed, all cultures were initiated with similar fibroblast numbers. Under the conditions of assay, proliferation is minimal (35). Moreover, at the conclusion of the experiment, DNA content was similar for OVA-challenged mouse fibroblast and control fibroblast populated gels (data not shown).
Finally, α-smooth muscle actin expression, which is a feature that is associated with myofibroblast differentiation, was also increased in cells obtained from OVA-challenged airways. This suggests that the cells from OVA-challenged animals were more myofibroblastic than cells from control animals. This is consistent with an augmented repair phenotype, as myofibroblasts are generally more active in repair responses (19, 30).
TGF-β has been associated with expression of myofibroblast-like features in fibroblasts (22). Other conditions of culture, such as serum (36) or culture on a solid substrate (37) also induce α-SMA expression. Thus it is possible that the culture conditions resulted in some of the features observed in the cultured cells. However, differences between OVA-challenged and control mouse fibroblasts were readily detected. Moreover, in vitro exposure to exogenous TGF-β1 stimulated all of the bioassays assessed in the current study. The in vitro effects of TGF-β, however, have been reported to be transient in contrast to the persistent phenotypic alterations observed in cells obtained from patients with asthma and from animals (22), suggesting that the differences between OVA-challenged and control mouse cells are not the result of TGF-β production by the OVA-challenged mouse cells. In addition, in the current study, both control and OVA-challenged mouse cells demonstrated the ability to respond to exogenous TGF-β (100 pM). This concentration was selected as it is maximal or near maximal for many types of normal cells. It is possible that normal and OVA-challenged mouse cells could differ in their sensitivity to lower concentrations of TGF-β. While chronic TGF-β exposure may alter cellular phenotype, it is unlikely that the effects of TGF-β produced by the OVA-stimulated fibroblasts in the cultures are a sufficient explanation for the differences observed.
In summary, the present study supports the concept that phenotypically altered fibroblasts can contribute to airway remodeling in asthma. Fibroblasts cultured from the lungs of chronically OVA-sensitized and -challenged animals demonstrated consistently augmented repair responses for a number of functional assays. This was true both for tracheobronchial and distal lung fibroblasts. The ability of chronic OVA exposure to alter lung mesenchymal cell populations is an appealing mechanism that could account for the structural and functional alterations characterizing chronic asthma.
Supplementary Material
Acknowledgments
The authors thank Ms. Lillian Richards for the excellent secretarial support.
This work was supported by the Larson Endowment, University of Nebraska Medical Center, and by NHLBI grant R01 HL073739 (to S.I.R.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0089OC on June 15, 2007
Conflict of Interest Statement: S.I.R. has participated as a speaker in scientific meeting and courses under the sponsorship of AstraZeneca (AZ) and GlaxoSmithKline (GSK). He serves on advisory boards for Altana, AZ, Dey, GSK, and Inspire. He has conducted clinical trials for AZ, Centocor, GSK, Pfizer, Roche, and Sanofi. He has served as a consultant for AZ, GSK, Novartis, Pfizer, and Roche. A patent is pending on the use of PDE4 inhibitors in repair; S.I.R. is a co-inventor of the patent owned by the University of Nebraska Medical Center. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Djukanovic R, Roche WR, Wilson JW, Beasley CRW, Twentyman OP, Howarth PH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis 1990;142:434–457. [DOI] [PubMed] [Google Scholar]
- 2.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 1999;104:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med 1999;160:1035–1042. [DOI] [PubMed] [Google Scholar]
- 4.Hastie AT, Kraft WK, Nyce KB, Zangrilli JG, Musani AI, Fish JE, Peters SP. Asthmatic epithelial cell proliferation and stimulation of collagen production: human asthmatic epithelial cells stimulate collagen type III production by human lung myofibroblasts after segmental allergen challenge. Am J Respir Crit Care Med 2002;165:266–272. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PR, Burgess JK, Underwood PA, Au W, Poniris MH, Tamm M, Ge Q, Roth M, Black JL. Extracellular matrix proteins modulate asthmatic airway smooth muscle cell proliferation via an autocrine mechanism. J Allergy Clin Immunol 2004;113:690–696. [DOI] [PubMed] [Google Scholar]
- 6.Plante S, Semlali A, Joubert P, Bissonnette E, Laviolette M, Hamid Q, Chakir J. Mast cells regulate procollagen I (alpha(1)) production by bronchial fibroblasts derived from subjects with asthma through IL-4/IL-4delta2 ratio. J Allergy Clin Immunol 2006;117:1321–1327. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig MS, Ftouhi-Paquin N, Huang W, Page N, Chakir J, Hamid Q. Mechanical strain enhances proteoglycan message in fibroblasts from asthmatic subjects. Clin Exp Allergy 2004;34:926–930. [DOI] [PubMed] [Google Scholar]
- 8.Hopfenspirger MT, Parr SK, Hopp RJ, Townley RG, Agrawal DK. Mycobacterial antigens attenuate late phase response, airway hyperresponsiveness, and bronchoalveolar lavage eosinophilia in a mouse model of bronchial asthma. Int Immunopharmacol 2001;1:1743–1751. [DOI] [PubMed] [Google Scholar]
- 9.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol 2004;172:5016–5023. [DOI] [PubMed] [Google Scholar]
- 10.Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol 2002;168:2516–2522. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai K, Ohno I, Imai K, Nawata J, Hayashi K, Okada S, Senoo H, Hattori T, Shirato K. The involvement of matrix metalloproteinases in basement membrane injury in a murine model of acute allergic airway inflammation. Clin Exp Allergy 2002;32:1527–1534. [DOI] [PubMed] [Google Scholar]
- 12.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol 1972;54:626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Tate L, Ertl RF, Kawamoto M, Mio T, Adachi Y, Romberger DJ, Koizumi S, Gossman G, Robbins RA, et al. Bronchial epithelial cells regulate fibroblast proliferation. Am J Physiol 1995;269:L377–L387. [DOI] [PubMed] [Google Scholar]
- 14.Mio T, Adachi Y, Romberger DJ, Spurzem JR, Ertl RF, Carnevali S, Rennard SI. Human bronchial epithelial cells modulate collagen gel contraction by fibroblasts. Am J Respir Crit Care Med 1995;151:A561. [DOI] [PubMed] [Google Scholar]
- 15.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med 1962;115:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiura H, Liu X, Kobayashi T, Togo S, Ertl RF, Kawasaki S, Kamio K, Wang XQ, Mao L, Shen L, et al. Reactive nitrogen species augment fibroblast-mediated collagen gel contraction, mediator production and chemotaxis. Am J Respir Cell Mol Biol 2006;34:592–599. [DOI] [PubMed] [Google Scholar]
- 17.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin, a glycoprotein from basement membranes. J Biol Chem 1979;254:9933–9937. [PubMed] [Google Scholar]
- 18.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 19.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1990;3:507–511. [DOI] [PubMed] [Google Scholar]
- 20.Boulet LP, Laviolett M, Urcotte H, Cartier A, Dugas M, Malo JL, Boutet M. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest 1997;112:45–52. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Kohyama T, Wang H, Zhu YK, Wen FQ, Kim HJ, Romberger DJ, Rennard SI. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am J Physiol 2002;282:L1049–L1056. [DOI] [PubMed] [Google Scholar]
- 22.Liu XD, Umino T, Ertl R, Veys T, Skold CM, Takigawa K, Romberger DJ, Spurzem JR, Zhu YK, Kohyama T, et al. Persistence of TGF-beta1 induction of increased fibroblast contractility. In Vitro Cell Dev Biol Anim 2001;37:193–201. [DOI] [PubMed] [Google Scholar]
- 23.Bergeron C, Hauber HP, Gotfried M, Newman K, Dhanda R, Servi RJ, Ludwig MS, Hamid Q. Evidence of remodeling in peripheral airways of patients with mild to moderate asthma: effect of hydrofluoroalkane-flunisolide. J Allergy Clin Immunol 2005;116:983–989. [DOI] [PubMed] [Google Scholar]
- 24.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 2003;111:1293–1298. [DOI] [PubMed] [Google Scholar]
- 25.Lewis CC, Chu HW, Westcott JY, Tucker A, Langmack EL, Sutherland ER, Kraft M. Airway fibroblasts exhibit a synthetic phenotype in severe asthma. J Allergy Clin Immunol 2005;115:534–540. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs K, Negri J, Klinnert M, Rosenwasser LJ, Borish L. Interleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthma. Am J Respir Crit Care Med 1998;158:1958–1962. [DOI] [PubMed] [Google Scholar]
- 27.Silverman ES, Palmer LJ, Subramaniam V, Hallock A, Mathew S, Vallone J, Faffe DS, Shikanai T, Raby BA, Weiss ST, et al. Transforming growth factor-beta1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med 2004;169:214–219. [DOI] [PubMed] [Google Scholar]
- 28.Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol 2002;27:526–535. [DOI] [PubMed] [Google Scholar]
- 29.Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol 1997;16:664–673. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 31.Kotaru C, Schoonover KJ, Trudeau JB, Huynh ML, Zhou X, Hu H, Wenzel SE. Regional fibroblast heterogeneity in the lung: implications for remodeling. Am J Respir Crit Care Med 2006;173:1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennard SI, Bitterman PB, Crystal RG. Response of the lower respiratory tract to injury: Mechanisms of repair of the parenchymal cells of the alveolar wall. Chest 1983;84:735–739. [DOI] [PubMed] [Google Scholar]
- 33.Rennard SI, Hunninghake GW, Bitterman P, Crystal RG. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci USA 1981;78:7147–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses HL, Coffey RJ, Leof EB, Lyons RM, Keski-Oja J. Transforming growth factor beta regulation of cell proliferation. J Cell Physiol Suppl 1987;5:S1–S7. [DOI] [PubMed] [Google Scholar]
- 35.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three dimensional collagen gel matrix. In Vitro Cell Dev Biol 1996;32:427–433. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Bushel PR, Kumar CC. Smooth muscle alpha-actin promoter activity is induced by serum stimulation of fibroblast cells. Biochem Biophys Res Commun 1993;190:1115–1121. [DOI] [PubMed] [Google Scholar]
- 37.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn 2005;233:706–720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.