Abstract
Interleukin (IL)-1, a proinflammatory cytokine, is expressed in the lung after ozone (O3) exposure. IL-1 mediates its effects through the type I IL-1 receptor (IL-1RI), the only signaling receptor for both IL-1α and IL-1β. The purpose of this study was to determine the role of IL-1RI in pulmonary responses to O3. To that end, wild-type, C57BL/6 (IL-1RI+/+) mice and IL-1RI–deficient (IL-1RI−/−) mice were exposed to O3 either subacutely (0.3 ppm for 72 h) or acutely (2 ppm for 3 h). Subacute O3 exposure increased bronchoalveolar lavage fluid (BALF) protein, interferon-γ–inducible protein (IP)-10, soluble tumor necrosis factor receptor 1 (sTNFR1), and neutrophils in IL-1RI+/+ and IL-1RI−/− mice. With the exception of IP-10, all outcome indicators were reduced in IL-1RI−/− mice. Furthermore, subacute O3 exposure increased IL-6 mRNA expression in IL-1RI+/+, but not IL-1RI−/− mice. Acute (2 ppm) O3 exposure increased BALF protein, IL-6, eotaxin, KC, macrophage inflammatory protein (MIP)-2, IP-10, monocyte chemotactic protein-1, sTNFR1, neutrophils, and epithelial cells in IL-1RI+/+ and IL-1RI−/− mice. For IL-6, eotaxin, MIP-2, and sTNFR1, there were small but significant reductions of these outcome indicators in IL-1RI−/− versus IL-1RI+/+ mice at 6 hours after exposure, but not at other time points, whereas other outcome indicators were unaffected by IL-1RI deficiency. These results suggest that IL-1RI is required for O3-induced pulmonary inflammation during subacute O3 exposure, but plays a more minor role during acute O3 exposure. In addition, these results suggest that the induction of IL-6 via IL-1RI may be important in mediating the effects of O3 during subacute exposure.
Keywords: bronchoalveolar lavage, interleukin-6, macrophage, neutrophil, soluble tumor necrosis factor receptor
CLINICAL RELEVANCE
IL-1RI, the only signaling receptor for IL-1α and IL-1β, is important for pulmonary inflammatory responses to subacute O3 exposure, likely through induction of IL-6. However, IL-1RI is less important for lung inflammatory responses to acute O3 exposure.
Exposure to ozone (O3), a principal component of photochemical smog and a powerful oxidant, exacerbates symptoms of respiratory diseases, including asthma and cystic fibrosis (1, 2). In addition, in both developed and developing countries, elevations in ambient O3 concentrations are associated with increased mortality (3–5). Consequently, it is important to understand the mechanistic basis by which the respiratory system responds to O3.
In humans, exposure to O3 causes substernal irritation, cough, and decrements in pulmonary function (6, 7). In many species studied, O3 causes airway hyperresponsiveness (AHR) to nonspecific bronchoconstrictors, such as methacholine (MCh) (6, 8–10). Inhalation of O3 also leads to pulmonary injury and inflammation, including epithelial cell sloughing, lung hyperpermeability, and neutrophil emigration into the airspaces (7, 8, 11–19). This inflammatory response is characterized by increased pulmonary expression of cytokines, including IL-6, as well as chemokines, including macrophage inflammatory protein (MIP)-2, KC, interferon-γ–inducible protein (IP)-10, monocyte chemotactic protein (MCP)-1, and eotaxin. Indeed, in rodents, many of the pulmonary manifestations of O3 exposure have been attributed to the effects of these cytokines and chemokines. For example, interruption of IL-6, IP-10, KC, and MIP-2 signaling significantly diminishes O3-induced neutrophil emigration to the airspaces and/or epithelial cell injury in mice (15, 18–20). Genetic deficiency in CXCR2, the receptor for KC and MIP-2, also reduces O3-induced increases in respiratory system responsiveness to MCh (19).
Interleukin-1 (IL-1α and IL-1β) is also increased in lung tissue and alveolar macrophages after O3 exposure (16, 21–23), and exogenous administration of IL-1 has been shown to reproduce many of the characteristic responses to O3, including AHR, neutrophil emigration into the airspaces, and lung hyperpermeability (24, 25). Moreover, IL-1 has the capacity to induce the expression of many of the cytokines and chemokines (eotaxin, IL-6, IP-10, KC, MCP-1, and MIP-2) that are generated after O3 exposure in the lung (26–31). Hence, it is possible that induction of IL-1 by O3 leads to generation of these other cytokines and chemokines and to the ensuing pathology.
IL-1α and IL-1β have two cell surface receptors to which they can bind, the type I and II IL-1 receptor (IL-1RI and IL-1RII). Intracellular signaling cascades resulting in NF-κB activation and gene transcription occur only after IL-1RI activation, whereas IL-1 binding to IL-1RII results in no signal generation (32–34). The role of IL-1RI in the inflammatory response appears to be dependent upon the specific stimulus used. For example, IL-1RI deficiency alone ameliorates tissue injury and/or inflammation induced during glomerulonephritis, hepatic ischemia and reperfusion, or the administration of turpentine (35–38). However, pulmonary inflammation induced by stimuli including Escherichia coli, Streptococcus pneumoniae, and lipopolysaccharide is not diminished by IL-1RI deficiency (39–41).
Based on these observations, the purpose of this study was to examine the hypothesis that IL-1RI contributes to O3-induced pulmonary pathophysiology and cytokine and chemokine expression after O3 exposure. Park and colleagues recently reported that exogenous administration of an IL-1 receptor antagonist (IL-1Ra) attenuates O3-induced AHR, cytokine/chemokine expression, neutrophil emigration into the airspaces, and epithelial cell sloughing in mice (16). The authors studied responses to acute, high-dose O3 exposure (2 ppm for 3 h), but did not examine the role of IL-1 in responses to lower concentrations of O3 administered over a longer period of time (subacute exposure), an exposure protocol that more closely mimics typical environmental exposures. Kleeberger and colleagues have reported that murine responses to subacute and acute O3 exposure are determined by separate genetic loci (42). Thus, there is reason to believe that pulmonary responses to subacute and acute O3 exposure are differentially regulated. We sought to determine if IL-1RI contributed to either or both of these responses. Accordingly, we examined O3-induced pulmonary injury and inflammation after subacute O3 exposure (0.3 ppm for 72 h) in wild-type, C57BL/6 (IL-1RI+/+) mice and in mice genetically deficient in IL-1RI (IL-1RI−/−). To confirm the results of Park and coworkers (16), we also examined the effect of acute O3 exposure (2 ppm for 3 h) in IL-1RI+/+ and IL-1RI−/− mice.
MATERIALS AND METHODS
Animals
Male and female mice genetically deficient in IL-1RI (IL-1RI−/−) (43) were maintained and bred in a rodent barrier facility at the Harvard School of Public Health (Boston, MA). Because these animals were backcrossed onto a C57BL/6 background for at least five generations, age- and sex-matched C57BL/6J (IL-1RI+/+) mice, purchased from The Jackson Laboratory (Bar Harbor, ME) at 7 weeks of age, were used as controls. All mice were housed in micro-isolator cages within the facility, where they were given food and water ad libitum and exposed to a 12-hour light-dark cycle. The Harvard Medical Area Standing Committee on Animals approved all of the experimental procedures used in this study.
Protocol
To assess pulmonary injury and inflammation, separate cohorts of mice were subjected to either a subacute (0.3 ppm for 72 h) or acute (2 ppm for 3 h) O3 exposure. For subacute exposures, mice were killed, bronchoalveolar lavage was performed, and the lungs were harvested within 30 minutes after cessation of the exposure. For acute exposures, the animals were studied 3, 6, or 24 hours after removal from the exposure chamber. Genotype-matched, air-exposed controls were subjected to the same experimental protocol as their O3-exposed counterparts. In some mice, to determine the effect of IL-1RI deficiency on O3-induced changes in the pattern of breathing, we measured breathing frequency and end-expiratory pause (EEP) before and after the cessation of either subacute or acute O3 exposure by placing the animals in a whole-body plethysmograph (Buxco Electronics, Inc., Wilmington, NC), as described previously (13, 14).
O3 Exposure
IL-1RI+/+ and IL-1RI−/− mice received either a subacute (72 h) or an acute (3 h) O3 exposure. For subacute (72 h) exposure, the entire micro-isolator cage, with the exception of the micro-isolator top, containing conscious IL-1RI+/+ and IL-1RI−/− mice was placed inside a 145-L stainless steel and Plexiglas chamber, where the animals were exposed to 0.3 ppm O3. During subacute exposure, the animals had continuous access to food and water. For acute (3 h) O3 exposure, conscious mice were placed in individual wire mesh cages inside the exposure chamber and exposed to 2 ppm O3. The O3 doses and exposure times were chosen to allow comparison with other studies in mice (16, 17, 44–49). Furthermore, the nomenclature used to describe these O3 exposure regimens, acute and subacute, has been extensively used by others (16, 17, 44–49). For room air exposures, the experimental protocol was identical to the corresponding O3 exposure, except that a separate and identical exposure chamber dedicated to room air exposure was used. Exposure chamber conditions and O3 generation and monitoring were previously described (19).
Bronchoalveolar Lavage
The animals were prepared for bronchoalveolar lavage (BAL) as previously described (19). The lungs were lavaged twice with 1 ml of ice-cold lavage buffer, PBS containing 0.6 mM EDTA. During each lavage, the lavage buffer was instilled and retrieved twice, and both lavagates subsequently pooled and stored on ice. The lavagate was spun, the supernatant collected and stored at −80°C, and the total BAL fluid (BALF) cells and differentials were determined as previously described (19). The total BALF protein concentration was determined spectrophotometrically according to the Bradford protein assay procedure (Bio-Rad Laboratories, Hercules, CA). The concentrations of BALF eotaxin, IP-10, IL-6, KC, MCP-1, MIP-2, and soluble tumor necrosis factor receptor 1 (sTNFR1) were determined with either enzyme-linked immunosorbent assay (ELISA) kits or DuoSet ELISA development systems (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions. These outcome indicators were measured because their levels increase in BALF after O3 exposure and/or their expression has been reported to be regulated by IL-1 (18, 26–31). Consequently, it is plausible that their expression is altered by IL-1RI deficiency after O3 exposure. Finally, because there were no significant differences in any of the BALF outcome indicators examined in mice exposed to room air for 3 versus 72 hours, the data for all air-exposed mice were pooled.
RNA Extraction and Real-Time Reverse-Transcription PCR
For measurement of IL-1β and IL-1α mRNA expression, RNA was extracted from frozen lung tissue and purified, and reverse transcription (RT) performed as previously described (18, 50, 51). RT reactions were diluted with 30 μl of water and stored at −80°C. Quantitative real-time RT-PCR was performed using an iCycler iQ real-time PCR detection system and iQ SYBR Green supermix in accordance with the manufacturer's instructions (Bio-Rad Laboratories). Primer sets and product sizes for murine IL-1β and β-actin were previously described (18, 50). Primer sets and the product size for IL-1α were as follows: IL-1α (forward, 5′-CAA ACT GAT GAA GCT CGT CA-3′ and reverse, 5′-TCT CCT TGA GCG CTC ACG AA-3′ [225 bp]) (52). Generation of positive controls for construction of standard curves was performed as previously described (50). For each gene, analyzed, melting curve analysis yielded a single peak consistent with one PCR product. For IL-1β and IL-1α, changes in mRNA expression in response to O3 exposure were assessed relative to changes in β-actin mRNA transcript copy number. There was no difference in β-actin levels between room air– and O3-exposed mice.
For measurement of IL-6 and ICAM-1 mRNA, total lung RNA was extracted and purified using the RNeasy miniprotocol (Qiagen, Valencia, CA) and RNase-free DNase set (Qiagen). Real-time RT-PCR was performed using the iScript one-step RT-PCR kit for probes (Bio-Rad Laboratories) and the iCycler iQ real-time PCR detection system (Bio-Rad Laboratories). Primers and TaqMan probes were designed using the Beacon Designer software (PREMIER Biosoft International; Palo Alto, CA). The primers used were previously described (41). Fold-induction values (versus IL-1RI+/+, room-air exposed mice) were normalized to the content of 18S rRNA (53).
Statistical Analysis
The effect of IL-1RI deficiency and O3 on mRNA levels and BALF parameters, were assessed by factorial ANOVA. For the analyses on BALF cells, values were logarithmically transformed to conform to a normal distribution. The effect of O3 and IL-1RI deficiency on the pattern of breathing was assessed by repeated measures ANOVA. STATISTICA software (StatSoft, Tulsa, OK) was used to perform all statistical analyses. The results are expressed as the arithmetic mean ± SEM, except for BALF cells, which are expressed as the geometric mean ± SEM. n is the number of mice per treatment group. A P value less than 0.05 was considered significant.
RESULTS
Effect of Subacute and Acute O3 Exposure on IL-1α and IL-1β mRNA Expression
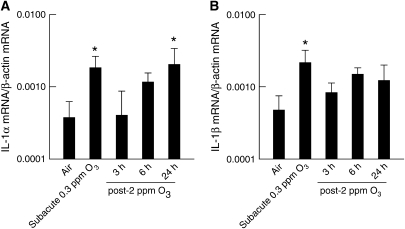
Real-time RT-PCR was used to determine IL-1α and IL-1β mRNA expression in the lungs of wild-type (IL-1RI+/+) mice after room air or O3 exposures (Figure 1). Transcripts for both IL-1α and IL-1β were expressed in the lungs of air-exposed, wild-type (IL-1RI+/+) mice. Subacute O3 exposure (0.3 ppm for 72 h) significantly increased the mRNA levels of both IL-1α and IL-1β when compared with their respective, air-exposed controls. After acute exposure to 2 ppm O3 for 3 hours, IL-1α mRNA expression was significantly increased only 24 hours after the cessation of O3 exposure, while IL-1β mRNA expression was unchanged at any time point.
Figure 1.
(A) IL-1α and (B) IL-1β mRNA expression in lung tissue from room air– or O3-exposed wild-type (C57BL/6) mice. Lungs were harvested 30 minutes after cessation of exposure to 0.3 ppm O3 for 72 hours, or 3, 6, or 24 hours after cessation of exposure to 2 ppm O3 for 3 hours. Lungs from air-exposed controls were obtained at the same time. mRNA transcript copy number was normalized to β-actin transcript copy number. n = 7–13 mice for each group. *P < 0.05 compared with air-exposed controls.
Effect of IL-1RI Deficiency on Pulmonary Injury and Inflammation after Subacute O3 Exposure
To assess the impact of IL-1RI deficiency on pulmonary injury and inflammation after subacute O3 exposure (0.3 ppm for 72 h), we examined BALF outcome indicators from IL-1RI+/+ and IL-1RI−/− mice. After room air exposure, the levels of BALF sTNFR1 as well as the number of BALF macrophages were significantly reduced in IL-1RI−/− versus IL-1RI+/+ mice (Figure 2). Subacute O3 exposure (0.3 ppm for 72 h) caused a significant increase in the concentrations of BALF protein, IP-10, sTNFR1, and the number of BALF neutrophils in both genotypes (Figure 2). However, the levels of BALF protein and sTNFR1 as well as the number of BALF neutrophils were significantly reduced in IL-1RI−/− compared with IL-1RI+/+ mice. Subacute O3 exposure also caused an increase in BALF macrophages in IL-1RI+/+, whereas no change was observed in IL-1RI−/− mice. There was no effect of this O3 exposure regimen or of genotype on the number of BALF epithelial cells. As we have previously reported that this O3 exposure regimen does not produce detectable levels of BALF IL-6 or KC (18), their levels were not measured.
Figure 2.
The concentrations of protein, interferon-γ–inducible protein (IP)-10, soluble tumor necrosis factor receptor 1 (sTNFR1), as well as the total number of macrophages, neutrophils, and epithelial cells in the bronchoalveolar lavage fluid (BALF) of wild-type, C57BL/6 (IL-1RI+/+; solid bars) and IL-1RI–deficient (IL-1RI−/−; open bars) mice 30 minutes after the cessation of a 72-hour exposure to either room air or 0.3 ppm ozone (O3). n = 8–26 for each treatment group. *P < 0.05 compared with genotype-matched, air-exposed controls. #P < 0.05 compared with IL-1RI−/− mice within the same exposure group.
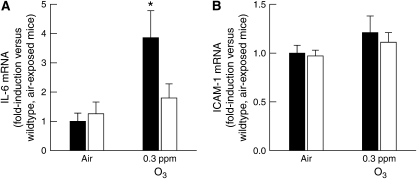
After subacute O3 exposure, BALF protein, sTNFR1, and neutrophils were reduced in IL-1RI−/− versus IL-1RI+/+ mice. Qualitatively similar results were observed in IL-6−/− versus IL-6+/+ mice after exposure to the same O3 exposure regimen (0.3 ppm O3 for 72 h) (18). Because IL-1 has the capacity to stimulate IL-6 expression (54), it is conceivable that the results observed in IL-1RI−/− mice were due to a reduced ability of IL-1RI−/− mice to produce IL-6. To examine this possibility, we measured IL-6 mRNA expression in IL-1RI+/+ and IL-1RI−/− mice after room air and subacute O3 exposure (Figure 3A). (Note that BALF IL-6 levels after these exposures are below the limit of detection of our assay [18].) After room air exposure, there was no difference in IL-6 mRNA expression between IL-1RI+/+ and IL-1RI−/− mice. However, subacute O3 exposure significantly increased IL-6 mRNA expression in IL-1RI+/+ but had no effect on IL-6 expression in IL-1RI−/− mice. In addition, intercellular adhesion molecule-1 (ICAM-1) expression is important in the migration of neutrophils to sites of inflammation in some settings (55). However, there was no effect of genotype or exposure on ICAM-1 mRNA expression (Figure 3B). Thus, IL-6, but not ICAM-1, was induced by subacute O3 exposure, and IL-6 expression required IL-1RI.
Figure 3.
(A) IL-6 and (B) intercellular adhesion molecule (ICAM)-1 mRNA expression in lung tissue from room air– or O3-exposed, wild-type C57BL/6 (IL-1RI+/+; solid bars) and IL-1RI–deficient (IL-1RI−/−; open bars) mice. Lungs were harvested 30 minutes after cessation of exposure to 0.3 ppm O3 for 72 hours. Lungs from air-exposed controls were obtained at the same time. mRNA levels for each treatment group were normalized for 18S rRNA and expressed as the fold-induction of the values determined for air-exposed, wild-type C57BL/6 (IL-1RI+/+) mice. n = 8–10 mice for each group. *P < 0.05 compared with air-exposed controls.
Effect of IL-1RI Deficiency on Pulmonary Injury and Inflammation after Acute O3 Exposure
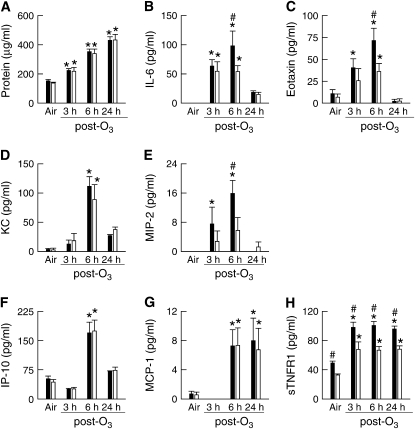
To assess the impact of IL-1RI deficiency on acute (2 ppm for 3 h) O3 exposure, we measured various BALF outcome indicators in IL-1RI+/+ and IL-1RI−/− mice 3, 6, and 24 hours after cessation of O3 exposure (Figures 4 and 5). For IL-1RI+/+ mice, the response to O3 followed two types of time courses depending on the outcome indicator. For IL-6, eotaxin, KC, MIP-2, and IP-10, BALF levels were increased above air-exposed controls at 3 and/or 6 hours after the cessation of O3 exposure and then began to resolve by 24 hours after exposure (Figure 4). For protein, MCP-1, and sTNFR1, levels after O3 exposure were elevated above air-exposed controls at either 3 and/or 6 hours after exposure and continued to remain elevated or even increased further at 24 hours after exposure. Compared with IL-1RI+/+ mice, IL-1RI deficiency resulted in decreased BALF sTNFR1 both in air- and O3-exposed mice. IL-1RI deficiency also resulted in a decrease in BALF IL-6, eotaxin, and MIP-2 6 hours after cessation of O3 but not at other times. There was no effect of IL-1RI deficiency on O3-induced changes in protein, KC, IP-10, or MCP-1.
Figure 4.
The concentrations of protein, IL-6, eotaxin, KC, macrophage inflammatory protein (MIP)-2, IP-10, MCP-1, and sTNFR1 in the BALF of wild-type, C57BL/6 (IL-1RI+/+; solid bars) and IL-1RI–deficient (IL-1RI−/−; open bars) mice 3, 6, or 24 hours after the cessation of a 3-hour exposure to either room air or 2 ppm ozone (O3). The concentrations of BALF protein, IP-10, and sTNFR1 from air-exposed mice are identical to those found in Figure 2 and are included here to demonstrate the effect of O3 on our outcome indicators. n = 5–26 for each treatment group. *P < 0.05 compared with genotype-matched, air-exposed controls. #P < 0.05 compared with IL-1RI−/− mice within the same exposure group.
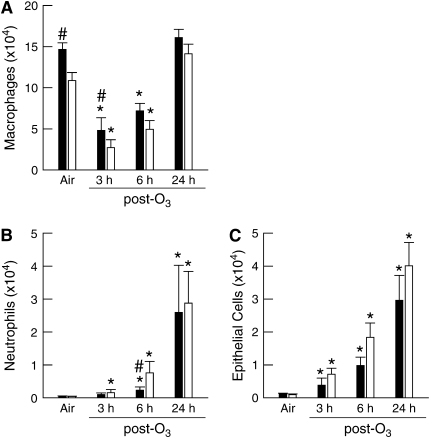
Figure 5.
The total number of macrophages, neutrophils, and epithelial cells in the BALF of wild-type, C57BL/6 (IL-1RI+/+; solid bars) and IL-1RI–deficient (IL-1RI−/−; open bars) mice 3, 6, or 24 hours after the cessation of a 3-hour exposure to either room air or 2 ppm ozone (O3). The data shown here from air-exposed mice are identical to those found in Figure 2 and are included here to demonstrate the effect of O3 on our outcome indicators. n = 8–26 for each treatment group. *P < 0.05 compared with genotype-matched, air-exposed controls. #P < 0.05 compared with IL-1RI−/− mice within the same exposure group.
In IL-1RI+/+ mice, BALF macrophages transiently decreased at 3 and 6 hours after O3 exposure before returning to pre-exposure levels at 24 hours after exposure (Figure 5), likely reflecting increased activation of macrophages and increased adhesion, making them less easy to wash out of the lungs. In contrast, BALF neutrophils and epithelial cells increased continuously from 3 to 24 hours after O3 exposure. IL-1RI deficiency resulted in a small, but significant, decrease in BALF macrophages in air-exposed mice, as noted earlier. A similar trend was observed at each time point after O3 exposure, but was significant only at 3 hours after exposure. IL-1RI deficiency did not reduce O3-induced changes in BALF neutrophils or epithelial cells. In fact, at 6 hours after O3 exposure, BALF neutrophils were actually increased in IL-1RI−/− versus IL-1R1+/+ mice.
Effect of IL-1RI Deficiency on O3-Induced Changes in the Pattern of Breathing
We have previously reported that exposure of mice to 2 ppm O3 for 3 hours results in a decrease in minute ventilation, particularly a marked reduction in breathing frequency resulting from an increased end-expiratory pause (EEP) (14). Genotype-related differences in the effect of O3 on breathing pattern could affect the inhaled dose of O3, since this dose is the product of minute ventilation, O3 concentration, and duration of exposure (56). Consequently, we used whole-body plethysmography to examine O3-induced changes in breathing frequency and EEP in IL-1RI+/+ and IL-1RI−/− mice. There was no difference in breathing frequency or EEP in air-exposed IL-1RI+/+ versus IL-1RI−/− mice (Table 1). Subacute O3 exposure had no effect on breathing pattern in mice of either genotype (Table 1). Breathing frequency was significantly decreased, and EEP was significantly increased in the 3-hour period immediately following cessation of exposure to 2 ppm O3 (Table 1), but there was no effect of genotype on either of these outcome indicators. Thus, it is unlikely that differences in the pattern of breathing account for the genotype-related differences in pulmonary injury and inflammation observed between IL-1RI−/− and IL-1RI+/+ mice.
TABLE 1.
EFFECT OF IL-1RI DEFICIENCY ON THE PATTERN OF BREATHING FOLLOWING SUBACUTE AND ACUTE ROOM AIR OR O3 EXPOSURE
| Genotype (Exposure) | Breathing Frequency (breaths/min) | End-Expiratory Pause (ms) |
|---|---|---|
| IL-1RI+/+ (air) | 345 ± 15 | 40.4 ± 5.2 |
| IL-1RI−/− (air) | 367 ± 13 | 29.4 ± 3.1 |
| IL-1RI+/+ (subacute 0.3 ppm O3) | 411 ± 12 | 25.9 ± 1.9 |
| IL-1RI−/− (subacute 0.3 ppm O3) | 359 ± 11 | 32.1 ± 5.3 |
| IL-1RI+/+ (acute 2 ppm O3) | 159 ± 15* | 236.7 ± 35.4* |
| IL-1RI−/− (acute 2 ppm O3) | 161 ± 16* | 223.0 ± 37.2* |
Results are the mean ± SEM for 7–24 mice in each group. Measurements were made between 30 and 45 minutes after the cessation of subacute room air or O3 (0.3 ppm) exposure and between 0.5 and 3 hours after the cessation of acute room air or O3 (2 ppm) exposure.
P < 0.05 compared with genotype-matched, air-exposed controls.
DISCUSSION
The results of this study indicate that deficiency of IL-1RI, the only signaling receptor for both IL-1α and IL-1β, attenuates O3-induced pulmonary injury and inflammation after subacute O3 exposure (0.3 ppm for 72 h) (Figure 2), while it has minimal effects on pulmonary injury and inflammation after acute O3 exposure (Figures 4 and 5). Furthermore, our data suggest that the amelioration of O3-induced pulmonary injury and inflammation in IL-1RI−/− mice may be due to reduced IL-6 expression in these animals.
Both IL-1α and IL-1β mRNA were expressed in the lungs of wild-type (IL-1RI+/+) mice after room air exposure (Figure 1). Changes in the expression of both IL-1α and IL-1β mRNA occurred with O3 exposure, but the effects were dependent on the nature of the O3 exposure regimen. For example, expression of IL-1α mRNA increased after both subacute and acute O3 exposure, whereas expression of IL-1β increased only after subacute O3 exposure. Consistent with these results, we have previously reported that acute O3 exposure (2 ppm for 3 h) had no effect on IL-1β mRNA expression measured 4 or 24 hours after the cessation of exposure (50). Macrophages are one source of IL-1 induced by O3 exposure (21), but other cells, including epithelial and smooth muscle cells, have the capacity to produce IL-1 (57–61), and it is conceivable that IL-1 is released by different cell types during acute versus subacute O3 exposure, accounting for the differences in IL-1 expression.
After subacute O3 exposure, IL-1RI deficiency significantly attenuated the levels of BALF protein and sTNFR1 as well as the number of BALF macrophages and neutrophils (Figure 2), suggesting that lung hyperpermeability and pulmonary inflammation are dependent upon IL-1RI signaling under these exposure conditions. We have previously reported that IL-6 deficiency, like IL-1RI deficiency, also diminishes subacute O3-induced increases in BALF protein, sTNFR1, and the number of BALF neutrophils (18). IL-1 has the capacity to stimulate IL-6 expression (54), and we have shown here that IL-1RI deficiency has the capacity to reduce the expression of IL-6 after subacute O3 exposure (Figure 3). Thus, it is conceivable that in the setting of subacute O3 exposure, the role of IL-1 may be to induce IL-6 expression and/or release, leading to reductions in the effects of IL-6 in IL-1RI−/− animals, rather than by IL-1 directly impacting outcome indicators such as BALF protein and neutrophils.
There were also small, but significant, decreases in the levels of BALF sTNFR1 and the number of BALF macrophages in IL-1RI−/− mice exposed to room air. These results suggest that IL-1RI influences either the expression and/or membrane cleavage of sTNFR1 in the lung in addition to the recruitment of macrophages to the airspaces under baseline conditions. However, the factor(s) mediating these effects are not known.
In contrast to subacute O3 exposure, our data suggest that IL-1RI signaling has a much more limited role in the pulmonary injury and inflammation observed following acute (3 h) O3 exposure. IL-6, eotaxin, and MIP-2 were significantly decreased in IL-1RI−/− mice versus IL-1RI+/+ mice (Figure 4), but the effects were only observed 6 hours after exposure and not at other time points, and changes in other inflammatory outcomes, including lung hyperpermeability, neutrophil recruitment, epithelial cell sloughing, KC, MCP-1, and IP-10, occurred independently of IL-1RI signaling. From the data presented, we cannot conclude whether the reductions in IL-6, eotaxin, and MIP-2 are a direct or an indirect result of IL-1RI deficiency. Curiously, even though there was evidence for a role of IL-1RI in the effects of 2 ppm O3 on IL-6, eotaxin, and MIP-2 at 6 hours, neither IL-1α nor IL-1β mRNA expression was increased by O3 exposure at this time. However, there was mRNA expression of both cytokines even in the air-exposed mice, suggesting that basal expression of IL-1 may be acting as a co-stimulus with some other factor that is induced by O3. We have also observed increased pulmonary IL-1RI mRNA expression in mice 4 hours after acute O3 exposure (2 ppm for 3 h) (unpublished microarray observations from our laboratory), a finding consistent with those of Park and colleagues (16). Thus, differences in the acute inflammatory response to O3 between IL-1RI+/+ and IL-1RI−/− mice (Figures 4 and 5) may be the result of basal levels of IL-1 signaling through increased IL-1RI. In addition, even though changes in mRNA were not observed at 6 hours (Figure 1), IL-1α and IL-1β protein levels could be elevated—for example, via changes in proteolytic or post-transcriptional processing and/or secretion. We did attempt to measure the levels of IL-1α and IL-1β in BALF after O3 exposure, but the levels of both cytokines were below the detection limits of our assay.
In contrast to the minimal role for IL-1RI in the response to acute 2 ppm O3 exposure observed in this study, Park and colleagues have reported that exogenous administration of IL-1ra attenuates increases in lung KC, MIP-2, TNF-α, epithelial cell sloughing, and neutrophils induced by the same exposure protocol (16). From these data, Park and colleagues concluded that IL-1 plays a substantial role in mediating pulmonary responses to acute 2 ppm O3 exposure. Park and colleagues reported increased expression of IL-1β, not IL-1α, mRNA with this protocol (16), whereas we observed the opposite (Figure 1). We do not know what accounts for this difference, but it is possible that the results reflect differences in other exposure conditions. Notably, the study of Park and coworkers (16) was performed at a high altitude (Denver, CO). It is conceivable that hypoxia per se or other climatic conditions (low humidity) may have contributed to the different outcomes. Furthermore, it is possible that the functional differences observed between our study and the study of Park and colleagues are a result of differences in IL-1 expression. That is, we observed an increase in IL-1α expression after acute (3 h) 2 ppm O3 exposure, whereas Park and colleagues observed an increase in IL-1β expression (16). The IL-1–dependent functional changes observed after O3 exposure in the study of Park and coworkers may only be dependent on increased IL-1β expression. Such changes would not be manifest in our study since we observed an increase in only IL-1α, and not IL-1β, expression.
As described above, we observed only a very limited role of IL-1RI in the pulmonary injury and inflammation observed after acute, high-dose O3 exposure (Figures 4 and 5). Previous reports also rule out major contributions from IL-6, TNF-α, and Toll-like receptor 4 in acute inflammatory responses to 2 ppm O3 (14, 17, 18, 47). Although abrogation of any one of these mediators alone has minimal or no effects on O3-induced pulmonary injury and inflammation, it is possible that they may be acting in concert to produce the inflammatory effects of O3. Supporting this postulate, whereas interrupting signaling from either IL-1 or TNF-α fails to compromise neutrophil recruitment elicited by Escherichia coli, Streptococcus pneumoniae, and lipopolysaccharide in the lungs, the combined interruption of both IL-1 and TNF-α signaling significantly decreases neutrophil recruitment elicited by any of these three stimuli (39, 62–64).
In conclusion, our data indicate that IL-1RI contributes prominently to the pulmonary inflammation observed after subacute O3 exposure (0.3 ppm for 72 h), whereas its role declines during acute (2 ppm) O3 exposure. Furthermore, IL-1RI induction of IL-6 may contribute to the pulmonary inflammation induced during subacute exposure.
Acknowledgments
The authors thank Mr. Michal M. Lupa, Mr. Benjamin T. Simms, Ms. Raya D. Terry, and Mr. Todd A. Theman for excellent technical assistance with this study.
This study was supported by National Heart, Lung, and Blood Institute Grants HL-33009 (to Jeffrey J. Fredberg) and HL-68153 (to J.P.M.), National Institute of Environmental Health Sciences Grants ES-013307 (to S.A.S.) and ES-00002 (to Joseph D. Brain), and an American Lung Association Research Training Fellowship RT-41-N (to R.A.J.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0315OC on June 15, 2007
Conflict of Interest Statement: S.A.S received $2,500 in lecture fees from GlaxoSmithKline in 2004. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med 2004;169:816–821. [DOI] [PubMed] [Google Scholar]
- 2.Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, Carlin BP, Klein M, Dorley J, Butler AJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol 2000;151:798–810. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Huang W, London SJ, Song G, Chen G, Jiang L, Zhao N, Chen B, Kan H. Ozone and daily mortality in Shanghai, China. Environ Health Perspect 2006;114:1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 2004;292:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neill MS, Loomis D, Borja-Aburto VH. Ozone, area social conditions, and mortality in Mexico City. Environ Res 2004;94:234–242. [DOI] [PubMed] [Google Scholar]
- 6.Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18–20 h postexposure to ozone. J Appl Physiol 2000;89:1804–1810. [DOI] [PubMed] [Google Scholar]
- 7.Hazucha MJ, Madden M, Pape G, Becker S, Devlin R, Koren HS, Kehrl H, Bromberg PA. Effects of cyclo-oxygenase inhibition on ozone-induced respiratory inflammation and lung function changes. Eur J Appl Physiol 1996;73:17–27. [DOI] [PubMed] [Google Scholar]
- 8.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 2003;95:938–945. [DOI] [PubMed] [Google Scholar]
- 9.Fedan JS, Millecchia LL, Johnston RA, Rengasamy A, Hubbs A, Dey RD, Yuan LX, Watson D, Goldsmith WT, Reynolds JS, et al. Effect of ozone treatment on airway reactivity and epithelium-derived relaxing factor in guinea pigs. J Pharmacol Exp Ther 2000;293:724–734. [PubMed] [Google Scholar]
- 10.DeLorme MP, Yang H, Elbon-Copp C, Gao X, Barraclough-Mitchell H, Bassett DJ. Hyperresponsive airways correlate with lung tissue inflammatory cell changes in ozone-exposed rats. J Toxicol Environ Health A 2002;65:1453–1470. [DOI] [PubMed] [Google Scholar]
- 11.Joad JP, McDonald RJ, Giri SN, Bric JM. Ozone effects on mechanics and arachidonic acid metabolite concentrations in isolated rat lungs. Environ Res 1994;66:186–197. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CJ, Stripp BR, Reynolds SD, Avissar NE, Reed CK, Finkelstein JN. Inflammatory and antioxidant gene expression in C57BL/6J mice after lethal and sublethal ozone exposures. Exp Lung Res 1999;25:81–97. [DOI] [PubMed] [Google Scholar]
- 13.Shore SA, Johnston RA, Schwartzman IN, Chism D, Krishna Murthy GG. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J Appl Physiol 2002;92:1019–1028. [DOI] [PubMed] [Google Scholar]
- 14.Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 2001;164:602–607. [DOI] [PubMed] [Google Scholar]
- 15.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol 2002;168:846–852. [DOI] [PubMed] [Google Scholar]
- 16.Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, Kim SH, Dinarello CA, Gelfand EW. IL-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol 2004;30:830–836. [DOI] [PubMed] [Google Scholar]
- 17.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am J Physiol Lung Cell Mol Physiol 2001;280:L537–L546. [DOI] [PubMed] [Google Scholar]
- 18.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 2005;288:L390–L397. [DOI] [PubMed] [Google Scholar]
- 19.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol 2005;288:L61–L67. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Zheng X, Witschi H, Pinkerton KE. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol Sci 2002;68:488–497. [DOI] [PubMed] [Google Scholar]
- 21.Arsalane K, Gosset P, Vanhee D, Voisin C, Hamid Q, Tonnel AB, Wallaert B. Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am J Respir Cell Mol Biol 1995;13:60–68. [DOI] [PubMed] [Google Scholar]
- 22.Ishii Y, Yang H, Sakamoto T, Nomura A, Hasegawa S, Hirata F, Bassett DJ. Rat alveolar macrophage cytokine production and regulation of neutrophil recruitment following acute ozone exposure. Toxicol Appl Pharmacol 1997;147:214–223. [DOI] [PubMed] [Google Scholar]
- 23.Pendino KJ, Shuler RL, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-alpha, and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol 1994;11:279–286. [DOI] [PubMed] [Google Scholar]
- 24.Patton LM, Saggart BS, Ahmed NK, Leff JA, Repine JE. Interleukin-1 beta-induced neutrophil recruitment and acute lung injury in hamsters. Inflammation 1995;19:23–29. [DOI] [PubMed] [Google Scholar]
- 25.Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1beta-induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol 2002;283:L909–L917. [DOI] [PubMed] [Google Scholar]
- 26.Jedrzkiewicz S, Nakamura H, Silverman ES, Luster AD, Mansharamani N, In KH, Tamura G, Lilly CM. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2000;279:L1058–L1065. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong DA, Major JA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol 2004;75:641–648. [DOI] [PubMed] [Google Scholar]
- 28.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, Banerjee A, McIntyre RC Jr. IL-1beta induces an exaggerated pro- and anti-inflammatory response in peritoneal macrophages of children compared with adults. Pediatr Surg Int 2004;20:238–242. [DOI] [PubMed] [Google Scholar]
- 29.Yan SR, Joseph RR, Wang J, Stadnyk AW. Differential pattern of inflammatory molecule regulation in intestinal epithelial cells stimulated with IL-1. J Immunol 2006;177:5604–5611. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, You S, Li W, Wang D, Nagpal ML, Mi Y, Liang P, Lin T. Expression and regulation of interferon-gamma-inducible protein 10 gene in rat Leydig cells. Endocrinology 1998;139:3637–3645. [DOI] [PubMed] [Google Scholar]
- 31.Kawano Y, Fukuda J, Itoh H, Takai N, Nasu K, Miyakawa I. The effect of inflammatory cytokines on secretion of macrophage colony-stimulating factor and monocyte chemoattractant protein-1 in human granulosa cells. Am J Reprod Immunol 2004;52:124–128. [DOI] [PubMed] [Google Scholar]
- 32.Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol 2002;14:117–122. [DOI] [PubMed] [Google Scholar]
- 33.Dayer JM. Evidence for the biological modulation of IL-1 activity: the role of IL-1Ra. Clin Exp Rheumatol 2002;20:S14–S20. [PubMed] [Google Scholar]
- 34.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol 2002; 20(5, Suppl 27)S1–13. [PubMed] [Google Scholar]
- 35.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG. Contributions of IL-1beta and IL-1alpha to crescentic glomerulonephritis in mice. J Am Soc Nephrol 2004;15:910–918. [DOI] [PubMed] [Google Scholar]
- 36.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol 1997;159:2452–2461. [PubMed] [Google Scholar]
- 37.Josephs MD, Solorzano CC, Taylor M, Rosenberg JJ, Topping D, Abouhamze A, Mackay SL, Hirsch E, Hirsh D, Labow M, et al. Modulation of the acute phase response by altered expression of the IL-1 type 1 receptor or IL-1ra. Am J Physiol Regul Integr Comp Physiol 2000;278:R824–R830. [DOI] [PubMed] [Google Scholar]
- 38.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol 2002;161:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol 2001;166:4042–4048. [DOI] [PubMed] [Google Scholar]
- 40.Moreland JG, Fuhrman RM, Wohlford-Lenane CL, Quinn TJ, Benda E, Pruessner JA, Schwartz DA. TNF-alpha and IL-1 beta are not essential to the inflammatory response in LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 2001;280:L173–L180. [DOI] [PubMed] [Google Scholar]
- 41.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis 2006;193:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleeberger SR, Levitt RC, Zhang LY. Susceptibility to ozone-induced inflammation: II. Separate loci control responses to acute and subacute exposures. Am J Physiol 1993;264:L21–L26. [DOI] [PubMed] [Google Scholar]
- 43.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol 1997;159:3364–3371. [PubMed] [Google Scholar]
- 44.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol 1998;274:L39–L46. [DOI] [PubMed] [Google Scholar]
- 45.Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol 2004;31:69–77. [DOI] [PubMed] [Google Scholar]
- 46.Jang AS, Choi IS, Yang SY, Kim YG, Lee JH, Park SW, Park CS. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration (Herrlisheim) 2005;72:79–84. [DOI] [PubMed] [Google Scholar]
- 47.Hollingsworth JW II, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med 2004;170:126–132. [DOI] [PubMed] [Google Scholar]
- 48.Tankersley CG, Kleeberger SR. Ozone-induced inflammation and altered ventilation in genetically susceptible mice: a comparison of acute and subacute exposures. Toxicol Lett 1994;72:279–289. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Gavett SH, Wills-Karp M. CD4+ T lymphocyte modulation of ozone-induced murine pulmonary inflammation. Am J Respir Cell Mol Biol 1995;12:396–403. [DOI] [PubMed] [Google Scholar]
- 50.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L856–L865. [DOI] [PubMed] [Google Scholar]
- 51.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 2005;115:103–109. [DOI] [PubMed] [Google Scholar]
- 52.Pournajafi Nazarloo H, Takao T, Taguchi T, Ito H, Hashimoto K. Modulation of type I IL-1 receptor and IL-1 beta mRNA expression followed by endotoxin treatment in the corticotropin-releasing hormone-deficient mouse. J Neuroimmunol 2003;140:102–108. [DOI] [PubMed] [Google Scholar]
- 53.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 2000;285:194–204. [DOI] [PubMed] [Google Scholar]
- 54.Faffe DS, Flynt L, Mellema M, Whitehead TR, Bourgeois K, Panettieri Jr RA, Silverman ES, Shore SA. Oncostatin M causes VEGF release from human airway smooth muscle: synergy with IL-1β. Am J Physiol Lung Cell Mol Physiol 2005;288:L1040–L1048. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal 2002;4:39–47. [DOI] [PubMed] [Google Scholar]
- 56.Weister MJ, Williams TB, King E, Menache MG, Muller FJ. Ozone uptake in awake Sprague-Dawley rats. Toxicol Appl Pharmacol 1987;89:429–437. [DOI] [PubMed] [Google Scholar]
- 57.Perrier S, Kherratia B, Deschaumes C, Ughetto S, Kemeny JL, Baudet-Pommel M, Sauvezie B. IL-1ra and IL-1 production in human oral mucosal epithelial cells in culture: differential modulation by TGF-beta1 and IL-4. Clin Exp Immunol 2002;127:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun 1993;61:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen WF, Fan W, Cao LX, Zhang PX. Multiple types of cytokines constitutively produced by an established murine thymic epithelial cell line. Eur Cytokine Netw 1992;3:43–52. [PubMed] [Google Scholar]
- 60.Shingu M, Nobunaga M, Ezaki I, Yoshioka K. Recombinant human IL-1 beta and TNF-alpha stimulate production of IL-1 alpha and IL-1 beta by vascular smooth muscle cells and IL-1 alpha by vascular endothelial cells. Life Sci 1991;49:241–246. [DOI] [PubMed] [Google Scholar]
- 61.Warner SJ, Auger KR, Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med 1987;165:1316–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mizgerd JP, Peschon JJ, Doerschuk CM. Roles of tumor necrosis factor receptor signaling during murine Escherichia coli pneumonia. Am J Respir Cell Mol Biol 2000;22:85–91. [DOI] [PubMed] [Google Scholar]
- 63.Mizgerd JP, Lupa MM, Hjoberg J, Valone J, Warren HB, Butler JP, Silverman ES. Roles for early response cytokines during Escherichia coli pneumonia revealed by mice with combined deficiencies of all signaling receptors for TNF and IL-1. Am J Physiol Lung Cell Mol Physiol 2004;286:L1302. [DOI] [PubMed] [Google Scholar]
- 64.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol 2005;175:7530–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]