Abstract
This study investigates the activity and inhibition resistance in excised rat lungs of a novel synthetic surfactant containing the phospholipase-resistant diether phosphonolipid DEPN-8 plus 1.5% bovine surfactant protein (SP)-B/C compared to calf lung surfactant extract (CLSE). DEPN-8 + 1.5% SP-B/C surpassed CLSE in normalizing surfactant-deficient pressure–volume (P–V) deflation mechanics in lavaged excised lungs in the presence of phospholipase A2 (PLA2) or C18:1 lyso-phosphatidylcholine (LPC). DEPN-8 + 1.5% SP-B/C had activity equal to CLSE in normalizing P–V mechanics in the absence of inhibitors or in the presence of serum albumin. These physiologic activity findings were directly consistent with surface activity measurements on the pulsating bubble surfactometer. In the absence of inhibitors, DEPN-8 + 1.5% SP-B/C and CLSE rapidly reached minimum surface tensions < 1 mN/m (0.5 and 2.5 mg surfactant phospholipid/ml). DEPN-8 + 1.5% SP-B/C maintained its high surface activity in the presence of PLA2, while the surface activity of CLSE was significantly inhibited by exposure to this enzyme. DEPN-8 + 1.5% SP-B/C also had greater surface activity than CLSE in the presence of LPC, and the two surfactants had equivalent surface activity in the presence of albumin. DEPN-8 + 1.5% SP-B/C also had slightly greater surface activity than CLSE when exposed to peroxynitrite in pulsating bubble studies. These results support the potential of developing highly active and inhibition-resistant synthetic exogenous surfactants containing DEPN-8 + apoprotein/peptide constituents for use in treating direct pulmonary forms of clinical acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS).
Keywords: synthetic lung surfactants, DEPN-8, phospholipase resistance, ALI/ARDS, surfactant dysfunction
CLINICAL RELEVANCE
This work involves the activity and inhibition resistance of a novel synthetic lung surfactant of potential utility for treating clinical states of lung surfactant deficiency and surfactant dysfunction (ALI/ARDS) in the future.
When endogenous lung surfactant is deficient or becomes dysfunctional in humans, it can be replaced by exogenous surface-active substitutes. Therapy with active exogenous surfactant drugs has proven to be life-saving in preventing and treating the respiratory distress syndrome (RDS) in premature infants, and ongoing research is focusing on extending surfactant therapy to pediatric and adult patients with clinical acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS) (see Refs. 1, 2 for review). Developing effective surfactant therapy for ALI/ARDS is particularly challenging, and requires the use of exogenous surfactants having maximal surface activity and the ability to resist inhibition from endogenous substances present in injured lungs as a result of permeability edema or inflammation.
The most active current clinical exogenous surfactant drugs are animal-derived as opposed to synthetic, but surfactant preparations synthesized in vitro have significant potential advantages as pharmaceutical products. This includes greater compositional reproducibility (less batch-to-batch variability) compared to animal surfactants, leading to easier and less costly manufacturing quality control. Synthetic surfactants are also not subject to concerns about prion-caused diseases like bovine spongioform encephalitis, and avoid ethnographic (cultural, religious) issues that affect animal-derived surfactants. Synthetic lung surfactants also can incorporate novel molecular components that are structurally resistant to phospholipase degradation and are designed to have favorable interfacial biophysical properties. Current exogenous lung surfactants all contain glycerophospholipids that can be degraded by phospholipases known to be present in the pulmonary interstitium or alveoli during inflammatory injury (3–10). Such degradation not only can deplete active surfactant lipids, but also generates chemical products like lysophosphatidylcholines (LPCs) and fluid free fatty acids that are severe biophysical inhibitors of surfactant activity (11–13).
Our prior research has synthesized and studied the surface activity of DEPN-8, a phospholipase-resistant C16:0 diether phosphonolipid that has enhanced adsorption and respreading properties compared to dipalmitoyl phosphatidylcholine (DPPC) (14–19). DEPN-8 has a gel to liquid-crystal transition temperature (Tc) of 45°C compared to 41°C for DPPC (18, 19), and forms tightly packed interfacial films able to reduce surface tension to < 1 mN/m during rapid compression (15–17). In addition, ether linkages between the fatty chains and glycerol backbone in DEPN-8 have greater flexibility than ester linkages in glycerophospholipids, facilitating adsorption and spreading. DEPN-8 also can form interdigitated bilayers, which may contribute to its improved respreading and adsorption relative to DPPC (19). Recent studies by Wang and coworkers (14) and Chang and colleagues (20) have shown that a model exogenous surfactant containing DEPN-8 + 1.5% bovine surfactant protein (SP)-B/C has very high surface activity and resistance to inhibition by phospholipase A2 (PLA2) in vitro. This work is extended here to define the physiologic activity and inhibition resistance of this surfactant in the excised, lavaged rat lung model of Bermel and coworkers (21). This rat lung model is the basis of an FDA-approved assay used in the quality control manufacture of two current clinical exogenous surfactant drugs in the United States (Survanta from Abbott/Ross Laboratories, N. Chicago, IL; and Infasurf from Forest Laboratories/ONY, Inc., Amherst, NY). Inhibitor compounds studied are PLA2, C18:1 lyso-phosphatidylcholine (LPC), bovine serum albumin (BSA), and peroxynitrite. Calf lung surfactant extract (CLSE), a clinically relevant and highly active animal-derived surfactant (2), is used as a comparative standard. The hypothesis tested is that DEPN-8 + 1.5% bovine SP-B/C will equal or exceed the physiological activity of CLSE in the excised lungs in correspondence with biophysical measurements of surface activity and inhibition resistance in vitro.
MATERIALS AND METHODS
Surfactant Substances
DEPN-8 [(±)-trimethyl(3-phosphonopropyl)ammonium, mono(2,3-bis(hexadecyloxy)propyl) ester] was synthesized and purified using our previously published methods (14, 15, 20). The chemical scheme for preparing DEPN-8 used the conversion of (±)-1-hexadecyloxy-2,3-propanediol to (±)-2,3-bis(hexadecyloxy)-1-propanol by way of hydroxyl protection at the 3-position, alkylation at the 2-hydroxyl group, and deprotection. Phosphonocholine placement involved treatment of (±)-2,3-bis(hexadecyloxy)-1-propanol with 3-bromopropylphosphono_di_chloridic acid prepared from 3-bromopropylphosphonic acid and PCl5 (14), followed by reaction with Me3N in CHCl3:MeOH:H2O (10:10:1). After concentration, the crude lipid was purified by exposure to Amberlite (Sigma-Aldrich, St. Louis, MO), flash chromatography with CHCl3:MeOH:H2O (60:35:5) as the elution solvent, and recrystallization from CHCl3/acetone (14). CLSE (a gift from ONY, Inc., Amherst, NY) was prepared by chloroform:methanol extraction of large aggregate surfactant lavaged from calf lungs as described by Wang and coworkers (22) and by Hall and colleagues (23, 24). SP-B/C were isolated from CLSE by gel permeation chromatography on a 1.5 × 50 cm column packed with Sephadex LH-20 (Pharmacia-LKB Biotechnology, Piscataway, NJ) with an elution solvent of 1:1 by volume chloroform and methanol plus 5% 0.1 N HCl (22, 24). Pooled fractions from the first and second column passes were extracted with chloroform:methanol (25) to remove acid. Final isolates of mixed protein contained only SP-B/C by gel electrophoresis (SDS- PAGE) and N-terminal amino acid analysis.
Inhibitors and Inhibition Conditions
Bovine serum albumin (BSA; Fraction V, Sigma Chemical, St. Louis, MO) or LPC (C18:1; Avanti Polar Lipids, Alabaster, AL) were incubated for 15 to 30 minutes at room temperature with surfactants (DEPN-8 + 1.5% SP-B/C or CLSE) dispersed in 0.15 M NaCl + 1.5 mM CaCl2 by probe sonication on ice (three 15-s bursts at 40 Watts power on a W220F Probe Sonicator, Plainview, NY) (12–14). BSA was used at a concentration of 3 mg/ml, and C18:1 LPC was present at 15% by weight relative to surfactant phospholipid. Phospholipase A2 (PLA2; Sigma Chemical) was incubated with dispersed surfactants for 30 minutes at 37°C (0.1 U/ml final enzyme concentration) (14). Peroxynitrite (> 90% pure; Cayman Chemical, Ann Arbor, MI) was stored in 0.3 M NaOH at −80°C, thawed carefully just before use, and incubated for 15 minutes at room temperature with dispersed surfactants in 10 mM HEPES (150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, and 10 μM Fe3+ EDTA, pH 7.4). Peroxynitrite concentration was 1 mM based on the Beer-Lambert Law (C = A/ɛl, where C is molar concentration, A is the measured absorbance at 302 nm, ɛ is the molar extinction coefficient of 1670 M−1cm−1, and l is the cuvette pathlength in cm).
Pulsating Bubble Surfactometer
Dynamic surface tension lowering was measured at a cycling rate of 20 cycles/minute at 37 ± 0.5°C on a pulsating bubble surfactometer (General Transco, Largo, FL). This instrument, based on the original design of Enhorning (26), gives a physiologically relevant assessment of overall surface activity that includes a combination of dynamic surface tension lowering and adsorption at physical conditions similar to those in the pulmonary alveoli. An air bubble, communicating with ambient air, was formed in 40 μl of dispersed surfactant held in a sample chamber mounted on a precision pulsator unit. The bubble was pulsated between maximum and minimum radii of 0.55 and 0.4 mm (50% area compression), and surface tension at minimum bubble radius (minimum surface tension) was calculated from the Laplace equation as a function of time of pulsation based on the measured pressure drop across the bubble interface (2, 26, 27). Surfactants were dispersed at a concentration of 0.5 or 2.5 mg phospholipid (phosphonolipid) per ml for bubble experiments. If present, inhibitor compounds (BSA, LPC, PLA2, or peroxynitrite) were at the concentrations noted above.
Excised Rat Lung Mechanical Studies
The excised rat lung model used to assess the physiologic activity and inhibition of exogenous surfactants has been detailed in prior work from our laboratory (11, 21, 23, 28–32). In brief, lungs from adult male rats (Sprague-Dawley, retired breeders, 300–500 g body weight) were surgically excised, vacuum degassed, and rapidly inflated to total lung capacity (TLC) at 30 cm H2O at 37°C. The lungs were stress-relaxed at TLC for 10 minutes, with air-leakage ruled out by a stringent requirement for < 0.1 ml/minute of injected air needed to maintain 30 cm H2O pressure at the end of this procedure. The lungs were then deflated at a slow rate of 2.47 ml/minute to define an initial “surfactant-sufficient” pressure–volume (P–V) deflation curve. After this, the excised lungs were depleted in endogenous surfactant by multiple lavages (15 lavages of 10 ml normal saline each), and a “surfactant-deficient” P–V deflation curve was determined using identical methods. Finally, an exogenous surfactant preparation was instilled via the trachea (100 mg/kg rat body weight instilled at a phospholipid [phosphonolipid] concentration of 35 mg/ml in 0.15 M NaCl), followed by measurement of a third P–V deflation curve to assess activity in improving mechanics in the presence or absence of inhibitors (albumin, LPC, PLA2). P–V data were plotted as lung volume as a fraction of TLC versus transpulmonary pressure. In addition, percent volume recoveries at selected transpulmonary pressures were calculated by the equation: 100 (Vsur − Vdef)/(Vnorm − Vdef), where Vnorm is the volume of the freshly excised lungs, Vdef is the volume of the lavaged surfactant-deficient lungs, and Vsur is the volume after surfactant instillation at the pressure of interest.
Data Analysis
All data are expressed as the mean ± 1 SEM. Statistical analyses used the Student's t test for comparisons of discrete data points, and functional data were analyzed by one-way ANOVA with Scheffe's post hoc analysis to adjust for multiple comparisons. Differences were considered statistically significant if P was < 0.05.
RESULTS
Dynamic Surface Tension Lowering and Inhibition Resistance of DEPN-8 + 1.5% Bovine SP-B/C and CLSE in Pulsating Bubble Studies
Although the surface activity and inhibition resistance of DEPN-8 + 1.5% SP-B/C has been reported previously by Wang and colleagues (14), analogous studies were done here on the specific DEPN-8 synthesis batch and bovine SP-B/C isolate used in physiologic experiments (Table 1). In addition to the three inhibitors examined later in excised rat lungs (BSA, C18:1 LPC, and PLA2), peroxynitrite was also assessed as a fourth inhibitor relevant for inflammatory lung injury. In the absence of inhibitor substances, DEPN-8 + 1.5% SP-B/C and CLSE had very high and similar overall surface activity. The activity of DEPN-8 + 1.5% SP-B/C was slightly better than CLSE at a low phospholipid concentration of 0.5 mg/ml (Table 1). At a higher surfactant concentration of 2.5 mg/ml, the surface activity of CLSE was greater than that of DEPN-8 + 1.5% SP-B/C, but both surfactants reached very low minimum surface tensions < 1 mN/m within 5 minutes of bubble pulsation (Table 1). The surface activity of DEPN-8 + 1.5% SP-B/C was completely resistant to inhibition from exposure to PLA2 (0.1 U/ml), while the surface activity of CLSE was severely inhibited by exposure to this enzyme at both surfactant concentrations studied. DEPN-8 (2.5 mg/ml) + 1.5% SP-B/C also had a greater resistance than CLSE (2.5 mg/ml) to biophysical inhibition from C18:1 LPC (Table 1). The two surfactants did not differ substantially in surface activity in the presence of BSA (Table 1). These results showing that DEPN-8 + 1.5% SP-B/C has superior surface activity to CLSE in the presence of PLA2 and LPC, and similar surface activity in the absence of inhibitors or in the presence of BSA, are consistent with the earlier findings of Wang and coworkers (14). In addition, DEPN-8 (2.5 mg/ml) + 1.5% SP-B/C also had a slightly greater inhibition resistance to peroxynitrite compared to CLSE at a surfactant concentration of 2.5 mg/ml (Table 1).
TABLE 1.
DYNAMIC SURFACE TENSION LOWERING OF DEPN-8 + 1.5% MIXED BOVINE SURFACTANT PROTEIN-B/C AND CALF LUNG SURFACTANT EXTRACT WITH AND WITHOUT INHIBITORS
| Surface Tension (mN/m) at Minimum Bubble Radius at Time (min)
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surfactant Mixture | 0.25 | 0.5 | 1 | 2 | 5 | 10 | 15 | 20 | ||||||||
| 0.5 mg/ml surfactant | ||||||||||||||||
| DEPN-8 + 1.5% SP-B/C | 13 ± 0 | 9 ± 1 | 6 ± 1 | 4 ± 1 | 1 ± 1 | < 1 | — | — | ||||||||
| + 0.1 U PLA2 | 16 ± 2 | 9 ± 1 | 6 ± 1 | 4 ± 1 | 1 ± 1 | < 1 | — | — | ||||||||
| + 15% (by wt) LPC | 35 ± 1 | 34 ± 1 | 31 ± 1 | 29 ± 1 | 27 ± 1 | 25 ± 1 | 22 ± 0 | 20 ± 0 | ||||||||
| + 3.0 mg/ml BSA | 48 ± 1 | 46 ± 1 | 40 ± 2 | 28 ± 1 | 22 ± 1 | 18 ± 1 | 11 ± 1 | 6 ± 1 | ||||||||
| + 1 mM peroxynitrite | 48 ± 1 | 45 ± 1 | 40 ± 1 | 32 ± 1 | 24 ± 1 | 20 ± 1 | 19 ± 1 | 18 ± 1 | ||||||||
| CLSE | 18 ± 0 | 15 ± 1 | 11 ± 2 | 9 ± 2 | 7 ± 2 | < 1 | — | — | ||||||||
| + 0.1 U PLA2 | 26 ± 2 | 24 ± 1 | 23 ± 1 | 23 ± 1 | 21 ± 1 | 20 ± 1 | 19 ± 0 | 19 ± 1 | ||||||||
| + 15% LPC | 35 ± 0 | 35 ± 0 | 31 ± 0 | 29 ± 0 | 27 ± 0 | 26 ± 0 | 25 ± 0 | 24 ± 0 | ||||||||
| + 3.0 mg/ml BSA | 46 ± 1 | 43 ± 1 | 36 ± 1 | 29 ± 2 | 22 ± 2 | 18 ± 1 | 12 ± 1 | 7 ± 1 | ||||||||
| + 1 mM peroxynitrite | 49 ± 1 | 44 ± 2 | 39 ± 1 | 33 ± 1 | 25 ± 1 | 22 ± 1 | 21 ± 1 | 20 ± 1 | ||||||||
| 2.5 mg/ml surfactant | ||||||||||||||||
| DEPN-8 + 1.5% SP-B/C | 12 ± 0 | 8 ± 1 | 6 ± 1 | 2 ± 0 | < 1 | — | ||||||||||
| + 0.1 U PLA2 | 15 ± 3 | 8 ± 1 | 5 ± 1 | 3 ± 0 | < 1 | — | ||||||||||
| + 15% LPC | 33 ± 2 | 30 ± 1 | 28 ± 1 | 20 ± 1 | 11 ± 1 | 4 ± 0 | < 1 | |||||||||
| + 3.0 mg/ml BSA | 21 ± 0 | 17 ± 1 | 13 ± 0 | 8 ± 0 | 4 ± 0 | < 1 | — | |||||||||
| + 1 mM peroxynitrite | 18 ± 1 | 15 ± 1 | 11 ± 1 | 7 ± 1 | 2 ± 1 | < 1 | — | |||||||||
| CLSE | 5 ± 2 | < 1 | — | — | — | — | — | — | ||||||||
| + 0.1 U PLA2 | 24 ± 2 | 23 ± 1 | 20 ± 1 | 19 ± 2 | 15 ± 1 | 14 ± 1 | 14 ± 1 | 14 ± 0 | ||||||||
| + 15% LPC | 34 ± 1 | 32 ± 1 | 29 ± 1 | 26 ± 1 | 19 ± 1 | 12 ± 1 | 5 ± 0 | <1 | ||||||||
| + 3.0 mg/ml BSA | 21 ± 1 | 17 ± 1 | 14 ± 0 | 10 ± 0 | 3 ± 0 | < 1 | — | — | ||||||||
| + 1 mM peroxynitrite | 22 ± 0 | 20 ± 1 | 18 ± 1 | 12 ± 2 | 5 ± 1 | < 1 | — | — | ||||||||
Definition of abbreviations: BSA, bovine serum albumin; CLSE, calf lung surfactant extract; LPC, C18:1 lysophosphatidylcholine; PLA2, phospholipase A2; SP, surfactant protein.
Data are mean ± SEM for n = 4–5. Surface tension at minimum radius (minimum surface tension) is shown as a function of time of pulsation on a bubble surfactometer (37°C, 20 cycles/min, 50% area compression, 0.5 or 2.5 mg phosphonolipid or phospholipid/ml). Mixed surfactant proteins SP-B/C were column purified from CLSE (see Materials and Methods).
Physiologic Activity of DEPN-8 + 1.5% SP-B/C and CLSE Instilled into Rat Lungs in the Absence of Inhibitors
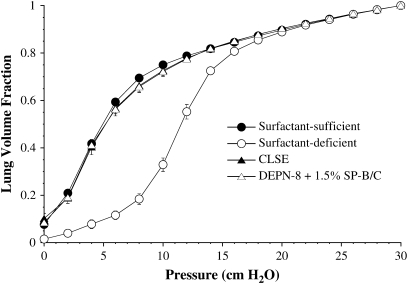
The P–V deflation mechanics of excised rat lungs in the surfactant-sufficient state (freshly excised), surfactant-deficient state (post-lavage), and after the instillation of exogenous surfactant in the absence of inhibitors are shown in Figure 1. Calculated percent volume recoveries at selected low transpulmonary deflation pressures are given in Table 2. Both DEPN-8 + 1.5% SP-B/C and CLSE at a tracheally instilled dose of 100 mg/kg rat bodyweight restored P–V mechanics almost completely to normal pre-lavage levels (Figure 1, Table 2). The equivalent activity of these two surfactants in improving P–V mechanics in surfactant-deficient excised rat lungs in the absence of inhibitors is consistent with their high and similar dynamic surface activity in Table 1.
Figure 1.
Effects of DEPN-8 + 1.5% surfactant protein (SP)-B/C in improving pressure–volume (P–V) deflation mechanics compared with calf lung surfactant extract (CLSE) in surfactant-deficient rat lungs. Lung volume is graphed as a fraction of total lung capacity at 30 cm H2O. Lungs were excised from adult rats, and mechanics were measured during quasistatic deflation in the normal freshly excised state (surfactant-sufficient), after depletion of endogenous surfactant by lavage (surfactant-deficient), and after tracheal instillation of surfactant at a dose of 100 mg phospholipid or phosphonolipid per kg rat bodyweight (see Materials and Methods). Calculated % volume recoveries at selected transpulmonary pressures are given in Table 2. Data are mean ± SEM for n = 6. There are no statistically significant differences between P–V curves for DEPN-8 + 1.5% SP-B/C and CLSE by ANOVA.
TABLE 2.
CALCULATED PERCENT VOLUME RECOVERIES AT LOW TRANSPULMONARY PRESSURES AFTER TRACHEAL INSTILLATION OF SURFACTANTS PLUS INHIBITORS INTO SURFACTANT-DEFICIENT EXCISED RAT LUNGS
| Lung Volume Recovery Percentage at Various Deflation at Various Transpulmonary Deflation Pressures (cm H2O)
|
||||
|---|---|---|---|---|
| Surfactant Mixture | 4 | 6 | 8 | 10 |
| DEPN-8 + 1.5% SP-B/C | 95.9 ± 3.0 | 94.6 ± 3.5 | 94.1 ± 4.7 | 98.3 ± 2.6 |
| CLSE | 95.9 ± 9.5 | 94.5 ± 6.7 | 93.7 ± 5.6 | 94.3 ± 5.0 |
| DEPN-8 + 1.5% SP-B/C + PLA2 | 93.2 ± 6.9* | 92.2 ± 5.4* | 93.3 ± 4.5* | 94.7 ± 4.0* |
| CLSE + PLA2 | 54.4 ± 6.1 | 59.8 ± 4.6 | 66.9 ± 4.3 | 72.3 ± 4.3 |
| DEPN-8 + 1.5% SP-B/C + LPC | 82.0 ± 1.1† | 81.2 ± 2.7† | 83.8 ± 1.9† | 84.8 ± 1.8† |
| CLSE + LPC | 64.7 ± 2.9 | 67.7 ± 3.3 | 71.8 ± 3.0 | 69.1 ± 2.1 |
| DEPN-8 + 1.5% SP-B/C + BSA | 53.7 ± 1.2 | 49.0 ± 1.0 | 55.6 ± 4.7 | 56.7 ± 2.9 |
| CLSE + BSA | 56.0 ± 2.9 | 51.9 ± 2.0 | 54.6 ± 2.5 | 55.4 ± 3.2 |
Definition of abbreviations: BSA, bovine serum albumin; CLSE, calf lung surfactant extract; LPC, C18:1 lysophosphatidylcholine; PLA2, phospholipase A2; SP, surfactant protein.
Percent recovery is calculated by the equation 100(Vsur − Vdef)/(Vnorm − Vdef), where Vnorm is the volume of the freshly excised lungs, Vdef is the volume of the lavaged surfactant-deficient lungs, and Vsur is the volume after surfactant instillation at the pressure of interest. PLA2 (0.1 U/ml), C18:1 LPC (15% by weight relative to lipid), or BSA (3 mg/ml) were incubated with surfactants in vitro before instillation into surfactant-deficient rat lungs (see Materials and Methods). Data are mean ± SEM for n = 3–6. Complete P–V deflation curves for the four cases in the table are shown in Figures 1–4, respectively.
Different from CLSE + PLA2, P < 0.0067.
Different from CLSE + LPC, P < 0.05.
Activity of DEPN-8 + 1.5% SP-B/C and CLSE in Excised Rat Lungs in the Presence of Inhibitor Compounds
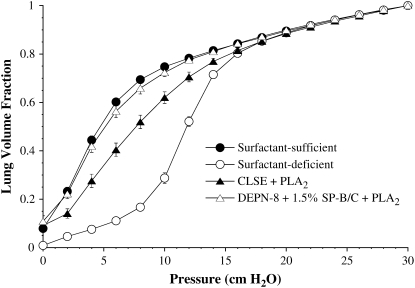
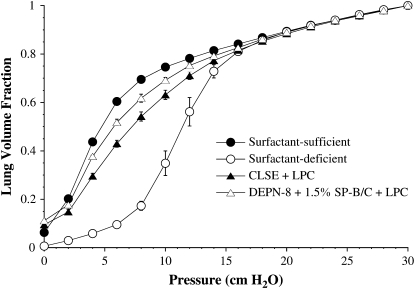
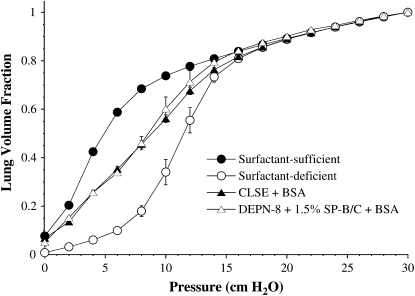
Figures 2–4 show the quasistatic deflation P–V mechanics of lavaged, excised rat lungs instilled with DEPN-8 + 1.5% SP-B/C or CLSE in the presence of either PLA2, BSA, or C18:1 LPC. Percent volume recoveries at low transpulmonary deflation pressures calculated from the P–V curves in these figures are given in Table 2. DEPN-8 + 1.5% SP-B/C maintained its full high physiologic activity in the excised lungs in the presence of PLA2 (Figure 2). In contrast, the ability of CLSE to restore normal P–V mechanics in rat lungs was significantly impaired by exposure to this lytic enzyme (Figure 2, P < 0.05 by ANOVA compared to DEPN-8 + 1.5% SP-B/C). Deflation lung volumes at transpulmonary pressures of 4, 6, 8, and 10 cm H2O were all significantly reduced for CLSE compared with DEPN-8 + 1.5% SP-B/C after exposure to PLA2 (Table 2, P < 0.0067). DEPN-8 + 1.5% SP-B/C also had greater physiologic activity in rat lungs than CLSE in the presence of C18:1 LPC (Figure 3, P < 0.05 by ANOVA). Deflation lung volumes at 4, 6, 8, and 10 cm H2O were all significantly greater for DEPN-8 + 1.5% SP-B/C than for CLSE in the presence of this inhibitory lipid (Table 2, P < 0.05). DEPN-8 + 1.5% SP-B/C and CLSE were equivalent in their ability to restore P–V deflation mechanics in the excised lungs in the presence of BSA (Figure 4, Table 2).
Figure 2.
Effects of DEPN-8 + 1.5% SP-B/C and CLSE on P–V deflation mechanics in surfactant-deficient rat lungs in the presence of PLA2. Surfactants were incubated in vitro with 0.1 U/ml of PLA2 for 30 min at 37°C before instillation into excised lavaged rat lungs. Other model details are as in the legend to Figure 1. Calculated % volume recoveries at selected transpulmonary pressures are given in Table 2. Data are mean ± SEM for n = 6. Differences between curves for DEPN-8 + 1.5% SP-B/C + PLA2 and CLSE + PLA2 are statistically significant by ANOVA (P < 0.05).
Figure 3.
Effects of DEPN-8 + 1.5% SP-B/C and CLSE on P–V deflation mechanics in surfactant-deficient rat lungs in the presence of C18:1 lysophosphatidylcholine (LPC). Surfactants were incubated in vitro with 15% by weight of C18:1 LPC for 15 to 30 minutes at room temperature before instillation into excised lavaged rat lungs. Other model details are as in the legend to Figure 1. Calculated % volume recoveries at selected transpulmonary pressures are given in Table 2. Data are mean ± SEM for n = 4. Differences between curves for DEPN-8 + 1.5% SP-B/C + 15% LPC and CLSE + 15% LPC are statistically significant by ANOVA (P < 0.05).
Figure 4.
Effects of DEPN-8 + 1.5% SP-B/C and CLSE on P–V deflation mechanics in surfactant-deficient rat lungs in the presence of bovine serum albumin (BSA). Surfactants were incubated in vitro with 3 mg/ml of BSA for 15 to 30 minutes at room temperature before instillation into excised lavaged rat lungs. Other model details are as in the legend to Figure 1. Calculated % volume recoveries at selected transpulmonary pressures are given in Table 2. Data are mean ± SEM for n = 3–4. There are no differences between P–V curves for DEPN-8 + 1.5% SP-B/C + BSA and CLSE + BSA by ANOVA.
DISCUSSION
In this paper, measurements of quasi-static P–V deflation mechanics in an excised rat lung model were used to define the physiologic activity of DEPN-8 + 1.5% SP-B/C in the presence and absence of physiologically relevant inhibitor substances (PLA2, serum albumin, and C18:1 LPC). The bovine lung surfactant extract CLSE, which is the substance of the clinical exogenous surfactant Infasurf, was used as a comparative standard of a highly active animal-derived exogenous surfactant drug substance. DEPN-8 + 1.5% SP-B/C surpassed CLSE in its ability to normalize P–V mechanics in the lavaged, excised lungs in the presence of PLA2 or C18:1 LPC (Figures 2 and 3, Table 2). DEPN-8 + 1.5% SP-B/C was equivalent to CLSE in its ability to restore P–V mechanics in the absence of inhibitors (Figure 1, Table 2) and in the presence of serum albumin (Figure 4, Table 2). These physiologic activity results found for DEPN-8 + 1.5% SP-B/C in surfactant-deficient rat lungs correlated very well with the high surface activity and inhibition resistance of this synthetic surfactant on the pulsating bubble surfactometer (Table 1 and as reported by Wang and colleagues [14]).
Although the present findings in excised rat lungs in situ need to be extended with additional studies in animal models of ALI/ARDS in vivo, they represent an important measure of surfactant physiologic activity in their own right. Theoretical understanding documents that pulmonary quasistatic P–V behavior is directly related to the surface-active function of lung surfactants (2). The specific ability of the excised rat lung model to define meaningful correlates between the surface and pulmonary activity of endogenous and exogenous lung surfactants has been extensively documented previously (11, 21, 23, 28–31). Biochemical measurements and thermodynamic analyses of P–V and surface tension–area behavior by Bermel and coworkers (21) have verified that the lavaged lungs in this model are surfactant-deficient and have increased surface tension forces. The lavage procedure does not alter lung tissue forces (21), and minimal liquid remains in the lungs after lavage (< 2 ml or 8% TLC). Surfactant-deficient mechanics in this model are highly reproducible, with recoil pressures increased relative to the freshly excised lungs at all volumes less than 90% TLC (11, 21, 23, 28–31). Activity findings from the excised rat lung model have also been shown to be highly consistent with physiologic data on surfactant replacement in premature lambs or in animals with surfactant dysfunction and acute lung injury in vivo (2, 31, 33–36). In particular, the excised rat lung model does not respond to the instillation of ineffective surfactant materials like pure DPPC that do not enhance pulmonary function in vivo (2), while P–V deflation mechanics improve to the normal level in a dose-dependent fashion upon instillation of lavaged bovine surfactant or CLSE (2, 11, 21, 23, 28–31) that are known to have high pulmonary activity in animal models in vivo (2, 31, 33–36).
The ability of DEPN-8 + 1.5% bovine SP-B/C to restore P–V mechanics in the excised lungs essentially to the full normal level in the absence of inhibitors (Figure 1) is impressive compared with several current clinical exogenous surfactants. The activity of DEPN-8 + 1.5% SP-B/C in excised rat lungs in the absence of inhibitors was equal to CLSE (Infasurf), which has been shown in a number of studies to have extremely high surface and physiologic activities that surpass the bovine-derived clinical surfactant Survanta (23, 28, 37, 38) and the synthetic clinical surfactant Exosurf (23). Survanta contains minimal levels of active SP-B (2, 37, 39), and only restores surfactant-deficient P–V mechanics to approximately half of the normal level in the absence of inhibitors in the same excised rat lung model as studied here (23, 28). Instilled Exosurf has even lower activity in improving mechanics in lavaged excised rat lungs (23). The ability of DEPN-8 + 1.5% SP-B/C to fully normalize rat lung P–V mechanics in the absence of inhibitors (Figure 1) also exceeds that previously reported in this model for Exosurf combined with 1.5% of column-purified bovine SP-B/C (23).
The very high surface and physiologic activity maintained by DEPN-8 + 1.5% SP-B/C in the presence of PLA2 (Table 1, Figure 2), and its relative resistance to LPC-induced inactivation (Table 1, Figure 3), are especially important characteristics for synthetic exogenous surfactants for possible use in ALI/ARDS. At a mechanistic level, a major factor in the resistance of DEPN-8 + 1.5% SP-B/C to activity inhibition from PLA2 is the molecular structural resistance of DEPN-8 to chemical degradation by this enzyme (14, 20). Phospholipases can be present in the pulmonary interstitium and alveoli during inflammatory lung injury (3–10), and PLA2 has been proposed as having specific importance in the pathogenesis of ARDS (5, 40). PLA2 is also present in meconium (41), and may contribute to the severe lung injury and respiratory failure found when this substance is aspirated by term infants at birth (42). PLA2 has also been shown to directly inhibit the surface activity of endogenous lung surfactant (4, 41, 43–45). As noted earlier, the chemical action of PLA2 in injured lungs not only can deplete active glycerophospholipids in endogenous and exogenous surfactants, but also produces LPC and fluid-free fatty acids that interact biophysically with intact surfactant to further impair surface activity (11–13). LPC and fluid fatty acids have both been shown to inhibit lung surfactant activity by mixing directly into the surface film and impairing its ability to reach low surface tensions during dynamic compression (11, 13). LPC and free fatty acids can also directly cause permeability injury at the level of the alveolocapillary membrane (46, 47).
The slightly increased resistance of DEPN-8 + 1.5% SP-B/C to surface activity inhibition by peroxynitrite compared to CLSE at a surfactant concentration of 2.5 mg/ml (Table 1) is also relevant for lung injury applications. Peroxynitrite is a highly reactive species that can be present in pulmonary tissue during the inflammatory response (e.g., 48–50). Previous studies by Haddad and coworkers (50) have shown that peroxynitrite can impair the surface activity of CLSE, and that this effect likely involves both surfactant lipids and apoproteins. Since DEPN-8 + 1.5% SP-B/C and CLSE both contain essentially the same content of hydrophobic surfactant proteins, the slightly improved surface activity of DEPN-8 (2.5 mg/ml) + 1.5% SP-B/C in the presence of peroxynitrite likely reflects an increased resistance of DEPN-8 to lipid peroxidation relative to the mix of saturated and unsaturated glycerophospholipids in CLSE.
DEPN-8 + 1.5% SP-B/C and CLSE were found to be equal in their ability to resist biophysical and physiologic inhibition by serum albumin (Tables 1 and 2, Figure 4). This in itself is significant, since CLSE has previously been shown to resist the inhibitory effects of albumin to a greater extent than other animal-derived exogenous surfactants including Survanta and Curosurf (2, 23, 37). For both DEPN-8 + 1.5% SP-B/C and CLSE, albumin-induced impairments in P–V mechanics in the excised lungs were found to be larger than those caused by C18:1 LPC (Table 2), whereas LPC had a greater inhibitory effect on surface activity compared with albumin on the pulsating bubble (Table 1). The reason for this behavior is unclear, but it does not alter the excellent overall conceptual correlation found between the relative biophysical and physiologic activities of DEPN-8 + 1.5% SP-B/C and CLSE when exposed to a given inhibitor (albumin, LPC, or PLA2). Albumin is known to act mechanistically to inhibit lung surfactant activity by competitive adsorption, which reduces or blocks the entry of active surfactant components into the air–water interface (2, 13). It may thus prove possible in the future to further improve the inhibition resistance of synthetic DEPN-8 surfactants to albumin by adding secondary novel lipids that increase adsorption.
A major goal of the current study was to progress toward developing synthetic lung surfactants that have equivalent or better overall surface activity and inhibition resistance compared with animal-derived surfactants, and are equal or superior as therapeutic agents when delivered to the pulmonary system. Our experiments used mixed bovine SP-B/C apoproteins separated chromatographically from lavaged surfactant and combined in mixtures with DEPN-8 as a proof of concept. However, fully synthetic lung surfactants ultimately will need to incorporate peptide components that are manufactured in vitro. Significant research effort has been directed at designing and synthesizing amphipathic peptides for use in exogenous lung surfactants, but replacing highly active native SP-B/C with synthetic substitutes has proven to be challenging (2, 51). The results of the present study indicate that fully synthetic surfactants of extremely high activity could be formulated by combining DEPN-8 with one or more synthetic peptides related to SP-B/C. Amphipathic synthetic peptides related to SP-B, which has the greatest activity of the native surfactant proteins in facilitating the surface activity of lipids (2, 28, 32, 52–58), are particularly important target compounds for use in synthetic surfactants. The present study did not examine the activity of specific synthetic peptides with DEPN-8, and this is an important area of future investigation.
There is a significant need for improved synthetic lung surfactants for treating clinical diseases of surfactant dysfunction and lung injury (ALI/ARDS). Aside from the pharmacologic advantages of synthetic lung surfactants noted at the beginning of this article (compositional reproducibility, easier quality control, no transmission of animal disease, and no animal-related ethnographic issues), another important potential benefit relates to material costs. On a body weight basis, adult doses ranging from 50 to 100 times larger than those used in premature infants are required to achieve an equivalent pulmonary amount of delivered drug. A dosage of 100 mg per kg of bodyweight translates to 5 to 10 g of administered surfactant in older children and adults, compared with 100 mg in a 1,000-g premature infant. Moreover, it can be expected that an increased average number of doses per patient may be necessary to mitigate the severe respiratory failure in patients with ALI/ARDS, further increasing total required drug amounts relative to neonatal RDS. Costs for animal-derived drugs are strongly dependent on animal usage, which is proportional to required drug amounts. A requirement for multiple 5- to 10-g doses of exogenous surfactant in patients with ALI/ARDS would make therapy very expensive if drug costs were even closely proportional to those currently charged for 100- to 200-mg vials of animal-derived surfactants in infants. The synthesis of surfactant drugs in vitro can be scaled up with much lower cost requirements compared with animal-derived products. Hopefully, the development of a highly active synthetic lung surfactant will provide significant future economic benefits for exogenous surfactant therapy in ALI/ARDS.
The complex pathophysiology of ALI/ARDS includes inflammation, vascular dysfunction, and cell/tissue injury in addition to surfactant dysfunction. However, surfactant dysfunction is an important contributor to respiratory failure in many affected patients, and ablating this aspect of pathology has the potential to provide crucial benefits to respiratory mechanics and function (see Refs. 1, 2 for review). Two surfactant drugs (Exosurf [59] and Survanta [60]) were tested in controlled trials in adults with sepsis-associated ARDS in the 1990s, with little or no beneficial effect on lung function or outcome. However, as discussed earlier, the surface activity, inhibition resistance, and pulmonary activity of these exogenous surfactants are not as high as CLSE (Infasurf), which was used in comparative studies here. Several studies in pediatric patients with acute respiratory failure have reported clinical benefits from therapy with instilled Infasurf (61–63). In particular, the recent blinded controlled multicenter trial of Willson and colleagues (63) in 153 infants, children, and young adults up to age 21 with ALI/ARDS demonstrated significantly improved survival in patients treated with Infasurf. The beneficial effects of this surfactant preparation were particularly pronounced in patients with direct pulmonary causes of ALI/ARDS (63). Results in the current study indicate that synthetic surfactants containing DEPN-8 + 1.5% SP-B/C or related peptides have the potential to be at least as active as CLSE (Infasurf) in reversing surfactant dysfunction in ALI/ARDS, although this needs to be investigated more fully in future work including animal experiments in vivo.
In summary, this paper has examined the physiologic activity and inhibition resistance of DEPN-8 + 1.5% bovine SP-B/C based on measurements of quasi-static P–V deflation mechanics in excised rat lungs. The physiologic activity of DEPN-8 + 1.5% SP-B/C in improving P–V mechanics in rat lungs was fully equal to that of CLSE in the absence of inhibitors and in the presence of serum albumin, and was superior to CLSE in the presence of PLA2 or C18:1 LPC. These physiologic findings correlated directly with biophysical assessments showing a similar relative pattern of surface activity and inhibition resistance for DEPN-8 + 1.5% SP-B/C compared with CLSE on the pulsating bubble surfactometer in vitro.
The financial support of the National Institutes of Health through grants HL-56176 and HL-66988 is gratefully acknowledged.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0434OC on June 7, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Chess P, Finkelstein JN, Holm BA, Notter RH. Surfactant replacement therapy in lung injury. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: mechanisms, pathophysiology, and therapy. Boca Raton: Taylor Francis Group, Inc; 2005. pp. 617–663.
- 2.Notter RH. Lung surfactants: basic science and clinical applications. New York: Marcel Dekker, Inc; 2000.
- 3.Kim DK, Fukuda T, Thompson BT, Cockrill B, Hales C, Bonventre JV. Bronchoalveolar lavage fluid phospholipase a2 activities are increased in human adult respiratory distress syndrome. Am J Physiol 1995;269:L109–L118. [DOI] [PubMed] [Google Scholar]
- 4.Holm BA, Kelcher L, Liu M, Sokolowski J, Enhorning G. Inhibition of pulmonary surfactant by phospholipases. J Appl Physiol 1991;71:317–321. [DOI] [PubMed] [Google Scholar]
- 5.Touqui L, Arbibe L. A role for phospholipase a2 in ards pathogenesis. Mol Med Today 1999;5:244–249. [DOI] [PubMed] [Google Scholar]
- 6.Vadas P. Elevated plasma phospholipase a2 levels: correlation with the hemodynamic and pulmonary changes in gram-negative septic shock. J Lab Clin Med 1984;104:873–881. [PubMed] [Google Scholar]
- 7.Kostopanagiotou G, Routs C, Smyrniotis V, Lekka ME, Kitsiouli E, Arkadopoulos N, Nakos G. Alterations in bronchoalveolar lavage fluid during ischemia-induced acute hepatic failure in the pig. Hepatology 2003;37:1130–1138. [DOI] [PubMed] [Google Scholar]
- 8.Attalah HL, Wu Y, Alaoui-El-Azher M, Thouron F, Koumanov K, Wolf C, Brochardz L, Harf A, Delclaux C, Touqui L. Induction of type-iia secretory phospholipase a2 in animal models of acute lung injury. Eur Respir J 2003;21:1040–1045. [DOI] [PubMed] [Google Scholar]
- 9.Nakos G, Kitsiouli E, Hatzidaki E, Koulouras V, Touqui L, Lekka ME. Phospholipases a2 and platelet-activating factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med 2003;33:772–779. [DOI] [PubMed] [Google Scholar]
- 10.Ackerman SJ, Kwatia MA, Doyle CB, Enhorning G. Hydrolysis of surfactant phospholipids catalyzed by phospholipase a2 and eosinophil lysophospholipases causes surfactant dysfunction: a mechanism for small airway closure in asthma. Chest 2003;123:255S. [DOI] [PubMed] [Google Scholar]
- 11.Hall SB, Lu ZR, Venkitaraman AR, Hyde RW, Notter RH. Inhibition of pulmonary surfactant by oleic acid: Mechanisms and characteristics. J Appl Physiol 1992;72:1708–1716. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Notter RH. Additivity of protein and non-protein inhibitors of lung surfactant activity. Am J Respir Crit Care Med 1998;158:28–35. [DOI] [PubMed] [Google Scholar]
- 13.Holm BA, Wang Z, Notter RH. Multiple mechanisms of lung surfactant inhibition. Pediatr Res 1999;46:85–93. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Schwan AL, Lairson LL, O'Donnell JS, Byrne GF, Foye A, Holm BA, Notter RH. Surface activity of a synthetic lung surfactant containing a phospholipase-resistant phosphonolipid analog of dipalmitoyl phosphatidylcholine. Am J Physiol 2003;285:L550–L559. [DOI] [PubMed] [Google Scholar]
- 15.Turcotte JG, Lin WH, Pivarnik PE, Sacco AM, Bermel MS, Lu Z, Notter RH. Chemical synthesis and surface activity of lung surfactant phospholipid analogs: II. Racemic n-substituted diether phosphonolipids. Biochim Biophys Acta 1991;1084:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Lu RZ, Turcotte JG, Notter RH. Dynamic interfacial properties of surface-excess films of phospholipids and phosphonolipid analogs: I. Effects of pH. J Colloid Interface Sci 1994;167:378–390. [Google Scholar]
- 17.Liu H, Turcotte JG, Notter RH. Dynamic interfacial properties of surface-excess films of phospholipid and phosphonolipid analogs: II. Effects of chain linkage and headgroup structure. J Colloid Interface Sci 1994;167:391–400. [Google Scholar]
- 18.Liu H, Turcotte JG, Notter RH. Thermotropic behavior of structurally-related phospholipids and phosphonolipid analogs of lung surfactant glycerophospholipids. Langmuir 1995;11:101–107. [Google Scholar]
- 19.Skita V, Chester DW, Oliver CJ, Turcotte JG, Notter RH. Bilayer characteristics of a diether phosphonolipid analog of the major lung surfactant glycerophospholipid dipalmitoyl phosphatidylcholine. J Lipid Res 1995;36:1116–1127. [PubMed] [Google Scholar]
- 20.Chang Y, Wang Z, Schwan AL, Wang Z, Holm BA, Baatz JE, Notter RH. Surface properties of sulfur- and ether-linked phosphonolipids with and without purified hydrophobic lung surfactant proteins. Chem Phys Lipids 2005;137:77–93. [DOI] [PubMed] [Google Scholar]
- 21.Bermel MS, McBride JT, Notter RH. Lavaged excised rat lungs as a model of surfactant deficiency. Lung 1984;162:99–113. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Hall SB, Notter RH. Dynamic surface activity of films of lung surfactant phospholipids, hydrophobic proteins, and neutral lipids. J Lipid Res 1995;36:1283–1293. [PubMed] [Google Scholar]
- 23.Hall SB, Venkitaraman AR, Whitsett JA, Holm BA, Notter RH. Importance of hydrophobic apoproteins as constituents of clinical exogenous surfactants. Am Rev Respir Dis 1992;145:24–30. [DOI] [PubMed] [Google Scholar]
- 24.Hall SB, Wang Z, Notter RH. Separation of subfractions of the hydrophobic components of calf lung surfactant. J Lipid Res 1994;35:1386–1394. [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–917. [DOI] [PubMed] [Google Scholar]
- 26.Enhorning G. Pulsating bubble technique for evaluation of pulmonary surfactant. J Appl Physiol 1977;43:198–203. [DOI] [PubMed] [Google Scholar]
- 27.Hall SB, Bermel MS, Ko YT, Palmer HJ, Enhorning GA, Notter RH. Approximations in the measurement of surface tension with the oscillating bubble surfactometer. J Appl Physiol 1993;75:468–477. [DOI] [PubMed] [Google Scholar]
- 28.Notter RH, Wang Z, Egan EA, Holm BA. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids 2002;114:21–34. [DOI] [PubMed] [Google Scholar]
- 29.Holm BA, Notter RH. Effects of hemoglobin and cell membrane lipids on pulmonary surfactant activity. J Appl Physiol 1987;63:1434–1442. [DOI] [PubMed] [Google Scholar]
- 30.Holm BA, Venkitaraman AR, Enhorning G, Notter RH. Biophysical inhibition of synthetic lung surfactants. Chem Phys Lipids 1990;52:243–250. [DOI] [PubMed] [Google Scholar]
- 31.Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL. Lung surfactant replacement in premature lambs with extracted lipids from bovine lung lavage: effects of dose, dispersion technique, and gestational age. Pediatr Res 1985;19:569–577. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Baatz JE, Holm BA, Notter RH. Content-dependent activity of lung surfactant protein b (sp-b) in mixtures with lipids. Am J Physiol 2002;283:L897–L906. [DOI] [PubMed] [Google Scholar]
- 33.Matalon S, Holm BA, Notter RH. Mitigation of pulmonary hyperoxic injury by administration of exogenous surfactant. J Appl Physiol 1987;62:756–761. [DOI] [PubMed] [Google Scholar]
- 34.Loewen GM, Holm BA, Milanowski L, Wild LM, Notter RH, Matalon S. Alveolar hyperoxic injury in rabbits receiving exogenous surfactant. J Appl Physiol 1989;66:1987–1992. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JJ, Holm BA, Hudak ML, Hudak BB, Ferguson WH, Egan EA. A controlled clinical comparison of four different surfactant preparations in surfactant-deficient preterm lambs. Am Rev Respir Dis 1992;145:999–1004. [DOI] [PubMed] [Google Scholar]
- 36.Egan EA, Notter RH, Kwong MS, Shapiro DL. Natural and artificial lung surfactant replacement therapy in premature lambs. J Appl Physiol 1983;55:875–883. [DOI] [PubMed] [Google Scholar]
- 37.Seeger W, Grube C, Günther A, Schmidt R. Surfactant inhibition by plasma proteins: Differential sensitivity of various surfactant preparations. Eur Respir J 1993;6:971–977. [PubMed] [Google Scholar]
- 38.Mizuno K, Ikegami M, Chen C-M, Ueda T, Jobe AH. Surfactant protein-b supplementation improves in vivo function of a modified natural surfactant. Pediatr Res 1995;37:271–276. [DOI] [PubMed] [Google Scholar]
- 39.Hamvas A, Cole FS, deMello DE, Moxley M, Whitsett JA, Colten HR, Nogee LM. Surfactant protein b deficiency: antenatal diagnosis and prospective treatment with surfactant replacement. J Pediatr 1994;125:356–361. [DOI] [PubMed] [Google Scholar]
- 40.Vadas P, Pruzanski W. Biology of disease: role of secretory phospholipases a2 in the pathobiology of disease. Lab Invest 1986;55:391–404. [PubMed] [Google Scholar]
- 41.Schrama AJJ, de Beufort AJ, Sukul YRM, Jansen SM, Poorthuis BJHM, Berger HM. Phospholipase a2 is present in meconium and inhibits the activity of pulmonary surfactant: an in vitro study. Acta Paediatr 2001;90:412–416. [PubMed] [Google Scholar]
- 42.Kaapa P. Meconium aspiration syndrome: a role for phospholipase a2 in the pathogenesis? Acta Paediatr 2001;90:365–367. [PubMed] [Google Scholar]
- 43.Enhorning G, Shumel B, Keicher L, Sokolowski J, Holm BA. Phospholipases introduced into the hypophase affect the surfactant film outlining a bubble. J Appl Physiol 1992;73:941–945. [DOI] [PubMed] [Google Scholar]
- 44.Duncan JE, Hatch GM, Belik J. Susceptibility of exogenous surfactant to phospholipase a2 degradation. Can J Physiol Pharmacol 1996;74:957–963. [PubMed] [Google Scholar]
- 45.Arbibe L, Koumanov K, Vail D, Rougeot C, Faure G, Havet N, Longacre S, Vargaftig BB, Voelker D, Wolf C, et al. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type ii phospholipase a2 and inhibited by a direct surfactant protein a-phospholipase a2 interaction. J Clin Invest 1998;102:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niewoehner D, Rice K, Sinha A, Wangensteen D. Injurious effects of lysophosphatidylcholine on barrier properties of alveolar epithelium. J Appl Physiol 1987;63:1979–1986. [DOI] [PubMed] [Google Scholar]
- 47.Hall SB, Notter RH, Smith RJ, Hyde RW. Altered function of pulmonary surfactant in fatty acid lung injury. J Appl Physiol 1990;69:1143–1149. [DOI] [PubMed] [Google Scholar]
- 48.Ischiropoulous H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys 1992;298:446–451. [DOI] [PubMed] [Google Scholar]
- 49.Davis IC, Lang JD, Matalon S. Roles of reactive oxygen and nitrogen species in lung injury. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: mechanisms, pathophysiology, and therapy. Boca Raton: Taylor & Francis Group; 2005. pp. 227–268.
- 50.Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol 1993;265:L555–L564. [DOI] [PubMed] [Google Scholar]
- 51.Johansson J, Gustafsson M, Zaltash S, Robertson B, Curstedt T. Synthetic surfactant protein analogs. Biol Neonate 1998;74:9–14. [DOI] [PubMed] [Google Scholar]
- 52.Seeger W, Günther A, Thede C. Differential sensitivity to fibrinogen inhibition of sp-c- vs. Sp-b-based surfactants. Am J Physiol 1992;261:L286–L291. [DOI] [PubMed] [Google Scholar]
- 53.Waring AJ, Walther FJ, Gordon LM, Hernandez-Juviel JM, Hong T, Sherman MA, Alonso C, Alig T, Braun A, Bacon D, et al. The role of charged amphipathic helices in the structure and function of surfactant protein b (sp-b). J Pept Res 2005;66:364–374. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Gurel O, Baatz JE, Notter RH. Differential activity and lack of synergy of lung surfactant proteins sp-b and sp-c in surface-active interactions with phospholipids. J Lipid Res 1996;37:1749–1760. [PubMed] [Google Scholar]
- 55.Yu SH, Possmayer F. Comparative studies on the biophysical activities of the low-molecular-weight hydrophobic proteins purified from bovine pulmonary surfactant. Biochim Biophys Acta 1988;961:337–350. [DOI] [PubMed] [Google Scholar]
- 56.Oosterlaken-Dijksterhuis MA, Haagsman HP, van Golde LM, Demel RA. Characterization of lipid insertion into monomolecular layers mediated by lung surfactant proteins sp-b and sp-c. Biochemistry 1991;30:10965–10971. [DOI] [PubMed] [Google Scholar]
- 57.Oosterlaken-Dijksterhuis MA, Haagsman HP, van Golde LM, Demel RA. Interaction of lipid vesicles with monomolecular layers containing lung surfactant proteins sp-b or sp-c. Biochemistry 1991;30:8276–8281. [DOI] [PubMed] [Google Scholar]
- 58.Oosterlaken-Dijksterhuis MA, van Eijk M, van Golde LMG, Haagsman HP. Lipid mixing is mediated by the hydrophobic surfactant protein sp-b but not by sp-c. Biochim Biophys Acta 1992;1110:45–50. [DOI] [PubMed] [Google Scholar]
- 59.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, Lemaire F, Long W, Zaccardelli DS, Pattishall EN, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. N Engl J Med 1996;334:1417–1421. [DOI] [PubMed] [Google Scholar]
- 60.Gregory TJ, Steinberg KP, Spragg R, Gadek JE, Hyers TM, Longmere WJ, Moxley MA, Guang-Zuan CAI, Hite RD, Smith RM, et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;155:109–131. [DOI] [PubMed] [Google Scholar]
- 61.Willson DF, Bauman LA, Zaritsky A, Dockery K, James RL, Stat M, Conrad D, Craft H, Novotny WE, Egan EA, et al. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Crit Care Med 1999;27:188–195. [DOI] [PubMed] [Google Scholar]
- 62.Willson DF, Jiao JH, Bauman LA, Zaritsky A, Craft H, Dockery K, Conrad D, Dalton H. Calf lung surfactant extract in acute hypoxemic respiratory failure in children. Crit Care Med 1996;24:1316–1322. [DOI] [PubMed] [Google Scholar]
- 63.Willson DF, Thomas NJ, Markovitz BP, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 2005;293:470–476. [DOI] [PubMed] [Google Scholar]