Abstract
Previously, we have identified the sequential activation of reactive oxygen species (ROS), mitochondria, and caspase-3, -8, and -9, in Siglec-8–mediated eosinophil apoptosis. Cytokine priming, which normally prolongs eosinophil survival, paradoxically potentiated this proapoptotic effect. The mechanisms of Siglec-8–mediated apoptosis after priming were therefore explored. Using IL-5 as the priming stimulus, the rate of Siglec-8–induced eosinophil apoptosis was found to be enhanced compared with unprimed cells, and mechanisms differed after IL-5 priming in that neither a pan-caspase inhibitor, nor a specific caspase-3 inhibitor, could override apoptosis. IL-5 priming also accelerated Siglec-8–mediated dissipation of mitochondrial membrane potential. Finally, both the mitochondrial electron transport inhibitor rotenone, and the ROS inhibitors diphenyleneiodonium and antimycin, completely inhibited Siglec-8–mediated apoptosis, even after IL-5 priming. These data demonstrate that IL-5 priming enhances Siglec-8–mediated mitochondrial and ROS-dependent eosinophil apoptosis and eliminates caspase dependence. The potential clinical implication of these findings is that cytokine priming, as often occurs in vivo in asthma and other hypereosinophilic disorders, may render eosinophils from such patients especially susceptible to the proapoptotic effects of a Siglec-8–engaging therapeutic agent.
Keywords: eosinophils, apoptosis, caspases, mitochondria, Siglec-8
CLINICAL REFERENCE
The implication of these findings is that cytokine priming, as occurs in asthma and other hypereosinophilic disorders, may render eosinophils from such patients especially susceptible to the proapoptotic effects of a Siglec-8–engaging therapeutic agent.
Siglec-8 is a member of the CD33 subfamily of the immunoglobulin gene superfamily of receptors (1). It is uniquely expressed on eosinophils, mast cells, and basophils (2). Although it exists as two splice variants, the consistent and predominant form contains two tyrosine-based cytoplasmic motifs implicated in cell signaling (3). Siglec-8 binds a unique carbohydrate structure, 6′ sulfated sialyl-Lewis X (4). Engagement of Siglec-8 on blood eosinophils results in caspase- and mitochondria-dependent apoptosis (5). In addition, previous studies have shown that eosinophil survival-promoting cytokines, such as IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF), fail to block apoptosis and instead enhance the sensitivity of eosinophils to undergo apoptosis in response to Siglec-8 engagement (6). In non–cytokine-primed eosinophils, Siglec-8 engagement induces activation of caspase-3, caspase-8, and caspase-9 (5). Based on experiments with pharmacologic inhibitors, caspases as well as mitochondria and reactive oxygen species (ROS) were identified as key constituents involved in Siglec-8–mediated apoptosis (5). Because cytokine priming occurs in vivo in a variety of allergic, parasitic, and other hypereosinophilic disorders (7), and because it enhances the pro-apoptotic effects of Siglec-8 engagement, the objective of the present studies was to identify the mechanisms of Siglec-8–mediated apoptosis after cytokine priming to determine how such treatment affects this apoptotic pathway.
MATERIALS AND METHODS
Antibodies, Reagents, and Recombinant Proteins
Siglec-8 immunoglobulin (Ig)G1 monoclonal antibody (mAb) 2E2 was generated as previously described (2). Goat anti-rabbit and donkey anti-mouse horseradish peroxidase–linked antibodies (Abs), polyclonal intact goat anti-mouse IgG, and mouse mAbs directed against FAS (CD95, IgM clone 7C11) or irrelevant IgG1 mAb were purchased from various commercial sources as previously described (5, 8). A selective caspase-3 inhibitor (Z-DEVD-FMK), as well as a pan-caspase inhibitor (Z-VAD-FMK), were obtained from EMD Chemicals (San Diego, CA), while diphenyleneiodonium (DPI), tetramethylrhodamine ethyl ester perchlorate (TMRE), carbonylcyanide m-chlorophenyl hydrazone (mCCP), rotenone, antimycin, and erythrosin-B were purchased from various commercial sources as previously delineated (5).
Eosinophil Purification and Culture
Written informed consent for blood donation or segmental allergen challenge followed by bronchoalveolar lavage (BAL) (the latter generously provided by Dr. Mark Liu, Johns Hopkins University, Baltimore, MD) was obtained using an institutional review board–approved university protocol. Eosinophils from allergic donors (and, where indicated, nonallergic donors) were purified from peripheral blood using density gradient centrifugation, erythrocyte hypotonic lysis, and immunomagnetic negative selection as described (8). Purity and viability were consistently greater than 99% as determined by erythrosin-B dye exclusion. Cells were cultured in RPMI 1640 medium with fetal calf serum and antibiotics as well as up to 30 ng/ml IL-5 as previously described (6). Eosinophils were harvested at different time points over 3 to 18 hours of co-culture with Siglec-8 mAb (2.5 μg/ml) in the presence or absence of polyclonal F(ab′)2 goat anti-mouse IgG Ab (10 μg/ml) used for secondary cross-linking, as previously described (6). In some experiments, cells were pre-incubated for 30 minutes at 37°C with IL-5, specific caspase inhibitors, or DPI as described (5) before addition of various Abs to the cultures.
Eosinophils obtained by bronchoalveolar lavage 18 to 24 hours after segmental allergen challenge of allergic subjects were enriched by density gradient centrifugation to at least 80% purity as previously described (9). Cells were cultured in RPMI 1640 medium with fetal calf serum and antibiotics with or without 30 ng/ml IL-5 for up to 48 hours or in the continued presence or absence of Siglec-8 mAb alone or an identical concentration of an isotype-matched IgG1 mAb.
Assessment of Apoptosis and Mitochondrial Membrane Potential
Eosinophil apoptosis was assessed following labeling with annexin-V and propidium iodide as described (6). To measure changes in mitochondrial membrane potential, cells were loaded for 30 minutes at 37°C with 100 nM TMRE, and reduction in TMRE staining was used as a marker of mitochondrial membrane potential loss (Δψm) (5). Separate aliquots of eosinophils in each culture were incubated with the mitochondrial membrane uncoupler mCCP (10 μM, 15 min, 37°C) as a positive control to induce maximal Δψm (10). Stained cells were then analyzed by flow cytometry (5).
Assessment of Caspase-3 Cleavage
Caspase-3 activity was measured fluorometically with the EnzCheck caspase-3 kit (Molecular Probes, Eugene, OR), as indicated by the manufacturer. Briefly, cell lysates were prepared by vortexing in 20 mM Tris-HCl (pH 7.2)/0.15 M NaCl/5 mM EDTA/5 mM benzamidine/0.5% Triton X-100/5 mM 2-mercaptoethanol. The peptidase activity of activated caspase-3 in the lysates was assayed using a caspase-3 substrate, Ac-DEVD-AMC, in the presence or absence of caspase-3 inhibitor Ac-DEVD-CHO, at 37°C (excitation at 400 nm, emission at 500 nm).
Statistical Analysis
Data are presented as mean ± SEM unless otherwise indicated. Statistical significance was determined either by ANOVA or Mann Whitney U-test, as appropriate. Values were considered significant at P < 0.05.
RESULTS
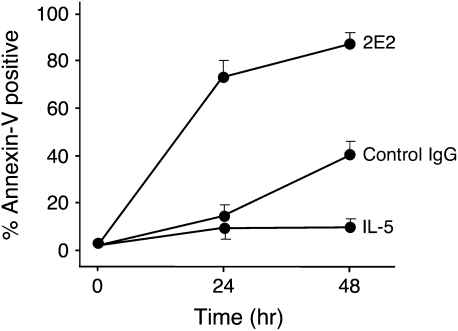
It was previously demonstrated that cytokine priming with IL-5 (or GM-CSF) enhanced the sensitivity of eosinophils to undergo apoptosis upon Siglec-8 crosslinking. This was initially demonstrated by showing a reduced amount of Siglec-8 mAb required for maximal apoptosis and the loss of need for secondary anti-mouse antibody crosslinking to induce apoptosis that is needed in freshly isolated, non–cytokine-primed eosinophils (6). Subsequently, comparable results were obtained using preparations of intravenous Ig containing naturally occurring anti–Siglec-8 antibodies (11). Therefore, initially, to more carefully explore the kinetics of this effect, eosinophils were incubated with or without IL-5 and then at various time points exposed to Siglec-8 mAb in the presence of secondary anti-mouse antibody. As shown in Figure 1, after 6 hours of incubation in the presence of IL-5, cells display exaggerated apoptotic responses. Similar results were obtained using eosinophils from nonallergic donors (data not shown). Although to a more modest degree, analogous results were also obtained after only 3 hours of culture (data not shown). In separate experiments using eosinophils isolated from allergic late phase BAL fluids, which have been exposed to priming cytokines in the allergic lung milieu (12), enhanced sensitivity to Siglec-8 mAb-mediated apoptosis was observed in that marked apoptosis was seen even in the absence of secondary cross-linking antibody (Figure 2).
Figure 1.
Siglec-8–induced eosinophil apoptosis is enhanced by IL-5 priming. Cells were co-cultured under Siglec-8 crosslinking conditions in the presence or absence of IL-5 (30 ng/ml). After 6 hours of co-incubation, cells were harvested and apoptosis was analyzed using annexin-V staining. Similar results (not shown) were obtained after 3 hours of culture (n = 6; *P < 0.05; **P < 0.005). 2E2, Siglec-8 mAb; 2°Ab, secondary polyclonal goat anti-mouse IgG.
Figure 2.
Eosinophils from late phase bronchoalveolar lavage fluids readily undergo apoptosis when exposed to Siglec-8 antibody alone. Eosinophils were incubated with IL-5 (30 ng/ml), Siglec-8 mAb (2.5 μg/ml, no secondary antibody), or an identical concentration of an isotype-matched IgG1 mAb as indicated, and apoptosis determined as in Figure 1. Results are means ± ranges of duplicate determinations from two experiments.
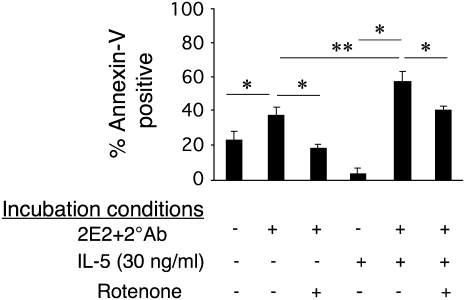
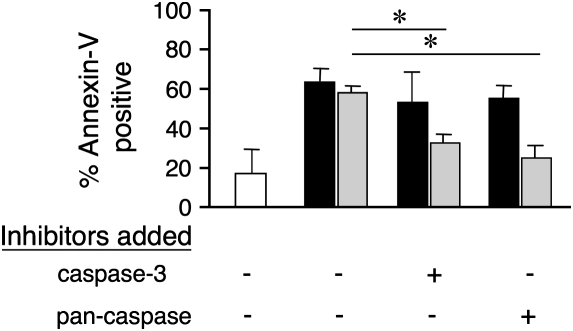
To explore the impact of IL-5 on mitochondrial injury pathways induced by Siglec-8 engagement, experiments were performed at both 3- and 18-hour time points in the presence or absence of IL-5, and Δψm as a marker of mitochondrial injury was monitored. As shown in Figure 3, at the 3-hour time point, IL-5 exposure resulted in accelerated Siglec-8–mediated Δψm, consistent with enhanced kinetics of mitochondrial injury. Although this difference is not seen at 18 hours, high levels of apoptosis with or without IL-5 priming are achieved by then, and are consistent with the finding that IL-5 does not override this pathway. As shown in Figure 4, apoptosis, even in the presence of IL-5, remains at least partially dependent on mitochondrial electron transport function because the apoptosis is significantly, albeit not completely, inhibited by rotenone, a mitochondrial electron transport inhibitor. However, unlike what is seen in freshly isolated non–cytokine-primed eosinophils (5), neither a caspase-3 inhibitor (Z-DEVD-FMK) nor a pan-caspase inhibitor (Z-VAD-FMK) had any effect on Siglec-8 mAb-mediated apoptosis under IL-5 priming conditions, even though they blocked FAS-induced apoptosis (Figure 5). However, as shown in Figure 6, cleavage of caspase-3 still occurs even in the presence of IL-5 priming, suggesting that cytokine priming does not eliminate Siglec-8–induced caspase cleavage. Finally, Figure 7 demonstrates that apoptosis after priming is still dependent on ROS because the ROS inhibitor DPI completely blocked apoptosis. Identical results were seen with another ROS inhibitor, antimycin (data not shown) and are consistent with previous data using DPI and N-acetyl cysteine, an ROS scavenger (11). Taken together, these data suggest that despite some evidence of continued caspase activity, the contribution of this pathway toward mediating Siglec-8–induced eosinophil death is negligible in the presence of IL-5. Thus, under cytokine priming conditions, Siglec-8–mediated apoptosis becomes predominantly or exclusively ROS and mitochondrial dependent.
Figure 3.
IL-5 does not override Siglec-8–mediated Δψm, and instead accelerates it. Cells were co-cultured under Siglec-8 crosslinking conditions in the presence or absence of IL-5 (30 ng/ml) for the times indicated. After cells were harvested, mitochondrial function was assessed using TMRE staining with MCCP used to generate maximal control staining. Reduced TMRE staining, a marker of increased mitochondrial dissipation, is seen (n = 3–6; *P < 0.05). Open bars, Siglec-8 mAb + secondary polyclonal goat anti-mouse IgG; solid bars, Siglec-8 mAb + secondary polyclonal goat anti-mouse IgG + IL-5.
Figure 4.
Depletion of mitochondrial electron transport function prevents Siglec-8–mediated eosinophil apoptosis: effect of IL-5. Cells were co-cultured as indicated in the presence or absence of the mitochondrial electron transport inhibitor rotenone (50 μM). After 18 hours, apoptosis was analyzed using annexin-V staining. Rotenone reversed Siglec-8–mediated apoptosis even in the presence of IL-5 (n = 3–7; *P < 0.05; **P < 0.005). 2E2, Siglec-8 mAb; 2°Ab, secondary polyclonal goat anti-mouse IgG.
Figure 5.
Neither a pan-caspase (Z-VAD-FMK) nor a caspase-3 inhibitor (Z-DEVD-FMK) had any effect on Siglec-8–mediated apoptosis under IL-5 (30 ng/ml) priming conditions, even though the latter do block FAS-induced apoptosis. Cells were preincubated with IL-5 for 24 hours before being co-cultured for an additional 24 hours in the presence of the indicated caspase inhibitors (45 μM) with or without Siglec-8 or FAS crosslinking. Apoptosis was then analyzed using annexin-V staining (n = 3–4; *P < 0.05). Open bars, IL-5 alone; solid bars, Siglec-8 mAb + secondary polyclonal goat anti-mouse IgG + IL-5; shaded bars, anti-CD95 mAb 7C11 + secondary polyclonal goat anti-mouse IgG + IL-5.
Figure 6.
IL-5 does not override Siglec-8–mediated caspase-3 cleavage in eosinophils. Cells were co-incubated with IL-5 (30 ng/ml) and the indicated reagents, cells were harvested after 18 hours of culture, and caspase-3 cleavage was assessed using the EnzyCheck assay. A caspase-3 inhibitor, Ac-DEVD-CHO, was also included in the experiments to document assay specificity (n = 3–5; *P < 0.005, **P < 0.05). Open bars, medium; solid bars, Siglec-8 mAb + secondary polyclonal goat anti-mouse IgG; shaded bars, Siglec-8 mAb + secondary polyclonal goat anti-mouse IgG + IL-5.
Figure 7.
Role of ROS in Siglec-8–mediated eosinophil apoptosis: effect of IL-5. Cells were cultured with or without IL-5 (30 ng/ml) for 18 hours as indicated. Apoptosis was analyzed using annexin-V staining. Incubation of cells with IL-5 along with Siglec-8 cross-linking significantly enhanced apoptosis compared with Siglec-8 cross-linking and control conditions. DPI, an inhibitor of ROS, reversed Siglec-8-mediated apoptosis even in the presence of IL-5 (n = 4–7; *P < 0.005, **P < 0.0005).
DISCUSSION
We and others have previously described the unanticipated observation that cytokine priming with IL-5 or GM-CSF enhanced sensitivity of eosinophils to undergo apoptosis induced by engagement of surface Siglec-8 (6, 11). This effect of IL-5 on eosinophil apoptosis was intriguing because IL-5 is normally considered an eosinophil survival–promoting cytokine and typically overrides most pro-apoptotic signals (13). In addition, this type of cytokine priming is felt to occur in vivo in asthma and other hypereosinophilic disorders and is associated not just with enhanced survival but also augmented degranulation and other secretory responses (14). In non–cytokine-primed eosinophils, Siglec-8 engagement induced apoptosis associated with the activation of caspases, along with involvement of mitochondria and ROS (5). In the present article we demonstrate that in the presence of IL-5, Siglec-8 engagement more readily initiates an apoptotic pathway through mitochondria and ROS. Based on the loss of the ability of either broad-spectrum or specific inhibitors of caspase-3 to block apoptosis, Siglec-8 appears to mediate apoptosis via an exaggerated role of ROS and mitochondrial injury, consistent with the known functions downstream of IL-5 priming, which includes enhanced ROS production by eosinophils (7).
Our findings best support a role for the intrinsic mitochondrial response pathway in Siglec-8–mediated eosinophil apoptosis even under priming conditions. Similar mechanisms of cell death have been described in other studies. For example, recent work by Lu and coworkers suggests that galectin-9 mediates both caspase-dependent and -independent death signaling in Jurkat cells downstream of mitochondria (15). Similarly, radiation damage initiates caspase-3–dependent and –independent cell death that is accompanied by the translocation of apoptosis-inducing factor (AIF) from the mitochondria to the nucleus (16). Because IL-5 priming is felt to occur in vivo in a variety of hypereosinophilic diseases, including asthma, it is tempting to speculate that the mechanisms of apoptosis observed in vitro in the present study may apply to how eosinophils would respond in vivo if exposed to Siglec-8 ligation. Our data showing enhanced susceptibility of in vivo–primed eosinophils obtained by BAL to undergo Siglec-8 mAb-induced apoptosis in vitro, along with data from von Gunten and colleagues showing exaggerated apoptosis after exposure of eosinophils from hypereosinophilic donors in vitro to preparations of intravenous Ig containing naturally occurring anti–Siglec-8 antibodies, are consistent with this hypothesis (11).
Data in the present study also support previous findings that Siglec-8 engagement activates the intrinsic stress-induced apoptotic signal pathway through generation of ROS and mitochondrial involvement rather than acting as a more traditional surface cell death receptor (5, 11). This conclusion is supported by the fact that Siglec-8 does not contain any known cytoplasmic signaling domains but does contain two tyrosine-based motifs, one of which appears to contain a conserved immunoreceptor tyrosine-based inhibitory motif (ITIM) that can potentially bind to Src-homology domain-containing phosphatases, such as SHP-1 and SHP-2 (1, 17). These are known to regulate functions of various cytokine receptors (18, 19) and therefore raise the possibility that Siglec-8 not only functions as an inhibitory receptor, but also regulates other functions, including perhaps the IL-5 receptor. Direct validation of this hypothesis will require additional studies. An alternative explanation of these results is that eosinophil activation via increased ROS production enhances Siglec-8–mediated eosinophil apoptosis, which is mediated by ROS and mitochondrial damage.
The selective expression of both Siglec-8 and IL-5 receptors on eosinophils suggests that the death pathway described here could be particularly important for, and unique to, eosinophils. In previous work, IL-5 has been described as an upstream inhibitor of apoptosis by targeting a pro-apoptotic Bcl-2 family member, namely Bcl-xl, thereby inhibiting mitochondrial responses and resulting in increased anti-apoptotic activity (20, 21). However, the pro-apoptotic mitochondrial effect seen with Siglec-8 ligation suggests that it can override the survival signal provided by IL-5. Indeed, after priming, this dissociation of IL-5–induced function from its usual downstream pro-survival regulatory pathway may represent a novel target that can be exploited therapeutically. It is also conceivable that the pro-apoptotic Bcl-2 family member, Bax, mediates mitochondrial release of cyt-c. A role for Bax is consistent with present data in that it does not require caspase activation for effect and occurs even in the presence of caspase inhibitors (22–25). Although it is not currently feasible to definitely discern between these specific functional pathways, it is clear that engagement of Siglec-8 on eosinophils in either normal or cytokine-primed eosinophils results in profound apoptosis and as such highlights the potential for Siglec-8–directed strategies to facilitate death and removal of eosinophils.
Acknowledgments
The authors thank Carol Bickel for expert technical assistance, and Dr. Mark Liu for providing late phase bronchoalveolar lavage eosinophils.
This work was supported by grant AI41472 from the National Institutes of Health. B.S.B. also received support as a Cosner Scholar in Translational Research from Johns Hopkins University. E.N.-B. was a recipient of a Rheumatoid Arthritis Foundation fellowship.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0154OC on August 9, 2007
Conflict of Interest Statement: B.S.B. and E.N.-B. are co-inventors on existing and pending Siglec-8–related patents. If Siglec-8–related products are developed in the future, under a licensing agreement between GlaxoSmithKline and the Johns Hopkins University, B.S.B. and E.N.-B. may be entitled to a share of royalties received by the University on the potential sales of such products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. S.A.H does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Varki A, Angata T. Siglecs: the major sub-family of i-type lectins. Glycobiology 2006;16:1R–27R. [DOI] [PubMed] [Google Scholar]
- 2.Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, Holmes SD, Abrahamson J, Hopson CB, Fischer EI, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol 2000;105:1093–1100. [DOI] [PubMed] [Google Scholar]
- 3.Nutku E, Aizawa H, Hudson SA, Bochner BS. Function of Siglec-8 on human eosinophils. Clin Exp Allergy Rev 2004;4:76–81. [Google Scholar]
- 4.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem 2005;280:4307–4312. [DOI] [PubMed] [Google Scholar]
- 5.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun 2005;336:918–924. [DOI] [PubMed] [Google Scholar]
- 6.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 2003;101:5014–5020. [DOI] [PubMed] [Google Scholar]
- 7.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006;24:147–174. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-FAS antibody treatment in vitro. Blood 1995;86:1437–1443. [PubMed] [Google Scholar]
- 9.Georas SN, Liu MC, Newman W, Beall WD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial activation accompany the recruitment of human granulocytes to the lung following segmental antigen challenge. Am J Respir Cell Mol Biol 1992;7:261–269. [DOI] [PubMed] [Google Scholar]
- 10.Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood 2001;98:2239–2247. [DOI] [PubMed] [Google Scholar]
- 11.von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, Simon HU. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol 2007;119:1005–1011. [DOI] [PubMed] [Google Scholar]
- 12.Liu MC, Xiao HQ, Lichtenstein LM, Huang SK. Prednisone inhibits TH2-type cytokine gene expression at sites of allergen challenge in subjects with asthma. 1995.
- 13.Simon HU. Molecules involved in the regulation of eosinophil apoptosis. Chem Immunol Allergy 2006;91:49–58. [DOI] [PubMed] [Google Scholar]
- 14.Bochner BS. Verdict in the case of therapies versus eosinophils: the jury is still out. J Allergy Clin Immunol 2004;113:3–9. [DOI] [PubMed] [Google Scholar]
- 15.Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji H, Nishi N, Hirashima M, Yamauchi A, Nakamura T. Characterization of Galectin-9-induced death of Jurkat T-cells. J Biochem (Tokyo) 2007;141:157–172. [DOI] [PubMed] [Google Scholar]
- 16.Hart LS, Ornelles DO, Koumenis C. The adenoviral e4orf6 protein induces atypical apoptosis in response to DNA damage. J Biol Chem 2007;282:6061–6067. [DOI] [PubMed] [Google Scholar]
- 17.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol 2005;5:431–437. [DOI] [PubMed] [Google Scholar]
- 18.Yu M, Luo J, Yang W, Wang Y, Mizuki M, Kanakura Y, Besmer P, Neel BG, Gu H. The scaffolding adapter Gab2, via SHP-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J Biol Chem 2006;281:28615–28626. [DOI] [PubMed] [Google Scholar]
- 19.Tortorella C, Simone O, Piazzolla G, Stella I, Antonaci S. Age-related impairment of GM-CSF-induced signalling in neutrophils: role of SHP-1 and SOCS proteins. Ageing Res Rev 2007;6:81–93. [DOI] [PubMed] [Google Scholar]
- 20.Dibbert B, Daigle I, Braun D, Schranz C, Weber M, Blaser K, Zangemeister-Wittke U, Akbar AN, Simon HU. Role for Bcl-xl in delayed eosinophil apoptosis mediated by granulocyte- macrophage colony-stimulating factor and interleukin-5. Blood 1998;92:778–783. [PubMed] [Google Scholar]
- 21.Huang CD, Wang CH, Liu CY, Lin SM, Chou CL, Liu WT, Lin HC, Kuo HP. Eosinophils from asthmatics release IL-5 in an autocrine fashion to prevent apoptosis through upregulation of Bcl-2 expression. J Asthma 2005;42:395–403. [DOI] [PubMed] [Google Scholar]
- 22.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC. Bax-induced cytochrome c release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol 1998;143:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xl. J Biol Chem 1999;274:2225–2233. [DOI] [PubMed] [Google Scholar]
- 24.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2001;2:63–67. [DOI] [PubMed] [Google Scholar]
- 25.Weinmann P, Gaehtgens P, Walzog B. Bcl-xl- and Bax-alpha-mediated regulation of apoptosis of human neutrophils via caspase-3. Blood 1999;93:3106–3115. [PubMed] [Google Scholar]