Abstract
The leaves of Lagerstroemia speciosa (Lythraceae), a Southeast Asian tree more commonly known as banaba, have been traditionally consumed in various forms by Philippinos for treatment of diabetes and kidney related diseases. In the 1990s, the popularity of this herbal medicine began to attract the attention of scientists worldwide. Since then, researchers have conducted numerous in vitro and in vivo studies that consistently confirmed the antidiabetic activity of banaba. Scientists have identified different components of banaba to be responsible for its activity. Using tumor cells as a cell model, corosolic acid was isolated from the methanol extract of banaba and shown to be an active compound. More recently, a different cell model and the focus on the water soluble fraction of the extract led to the discovery of other compounds. The ellagitannin Lagerstroemin was identified as an effective component of the banaba extract responsible for the activity. In a different approach, using 3T3-L1 adipocytes as a cell model and a glucose uptake assay as the functional screening method, Chen et al. showed that the banaba water extract exhibited an insulin-like glucose transport inducing activity. Coupling HPLC fractionation with a glucose uptake assay, gallotannins were identified in the banaba extract as components responsible for the activity, not corosolic acid. Penta-O-galloyl-glucopyranose (PGG) was identified as the most potent gallotannin. A comparison of published data with results obtained for PGG indicates that PGG has a significantly higher glucose transport stimulatory activity than Lagerstroemin. Chen et al. have also shown that PGG exhibits anti-adipogenic properties in addition to stimulating the glucose uptake in adipocytes. The combination of glucose uptake and anti-adipogenesis activity is not found in the current insulin mimetic drugs and may indicate a great therapeutic potential of PGG.
Keywords: diabetes, glucose transport, obesity, PGG, plant extract
Introduction
Type 2 diabetes has developed into a worldwide epidemic (1). Ironically, the dramatic increase in the prevalence of type 2 diabetes can be attributed to the rapid economic development and correlated to changes in lifestyle within the last 50 years. Type 2 diabetes is closely associated with obesity. Up to 90% of the patients in the US with type 2 diabetes are either overweight or obese (2,3). It seems likely that the readily available high calorie food and a sedentary life style are major causes for obesity. Obesity contributes to insulin resistance and type 2 diabetes. Reducing obesity and stopping weight gain constitutes a way to slow down the rate of occurrence of type 2 diabetes.
Type 2 diabetes is caused by insulin resistance, which is defined as defective insulin signaling and a decreased insulin efficiency to induce glucose transport from the blood into key target cells such as muscle and fat (adipocyte) cells (3). In general, obesity leads to hyperglycemia, which in turn leads to and exacerbates insulin resistance. Insulin resistance, if not treated, results in hyperinsulinemia and eventually leads to full blown type 2 diabetes (3,4). Obesity or excessive adiposity, particularly visceral adiposity, contributes to and worsens insulin resistance (2,5). Most antidiabetic drugs are hypoglycemic or anti-hyperglycemic (blood glucose level reducing). However, most of these drugs are, to different extents, weight gain promoting (adipogenic) (6,7). Thus, these drugs treat one of the key symptoms of type 2 diabetes, hyperglycemia, but exacerbate the condition of being overweight or obese, one of the leading causes of type 2 diabetes. Therefore, while these drugs are beneficial over the short term, they are not optimal for long term health of type 2 diabetic patient. The most desirable situation would be the development of new types of antidiabetic drugs that are either hypoglycemic or anti-hyperglycemic without the side effect of promoting weight gain (adiposity). Herbal medicines known to be useful in diabetes treatment may be able to lead to compounds with such a combination of ideal therapeutic properties (8–13).
Lagerstroemia speciosa
Lagerstroemia speciosa (Fig. 1), also called banaba in the Tagalog language of the Philippines, is a tropical plant found in many parts of Southeast Asia including the Philippines, Vietnam, Malaysia and southern China. It is a tree that can grow as tall as 20 m. Despite growing in several countries, only in the Philippines are the dried and shredded banaba leaves known to be used as a treatment for diabetes and kidney disease. It is not clear if banaba plants grown in different countries are equally effective in the treatment of diabetes.
Figure 1.
Langerstroemia speciosa. Tree and tree leaf.
Garcia published the first research on banaba's insulin-like, hypoglycemic effect as early as 1940 (14–18). Later, the popular use of banaba in the Philippines was noticed and led to its introduction in Japan. However, it was not until 50 years after Garcia's first publication that scientific interest in banaba's potential for the treatment of diabetes resurfaced. Scientists from countries including Japan, the Philippines, Korea and the United States are currently studying banaba. Lagerstroemia speciosa (banaba) has become relatively popular in the form of health-promoting tea products in Eastern Asia and the United States.
In 1996, Kakuda et al. (19) studied banaba's antidiabetic activity by preparing water and methanol extracts of the plant. After feeding the extracts to hereditary type 2 diabetic KK-Ay/Ta Jcl mice, they found that food containing either 5% of water extract (BE) or 3% of methanol extract was effective in reducing blood glucose and insulin levels (P < 0.05) (19). It is interesting to note that the total cholesterol in treated mice was also significantly reduced, but the plasma triglyceride level remained unchanged (19).
In a second study by Kakuda's research group, food containing 5% banaba water extract was used to feed female obese KK-Ay/Ta Jcl mice. Obese mice treated with banaba extract had a significantly reduced body weight (∼10%) compared with control mice fed with a regular diet (20). No change in food intake was observed (20). Interestingly, it was also discovered that liver triglyceride content was reduced by more than 40% in the banaba extract-treated mice. In addition, the parametrial adipose tissue was 10% lighter (P < 0.01) (20). However, as in the previous antidiabetic activity study of BE in animals (19), both the identity of the effective component(s) and the mechanism for the activity were not studied. Nevertheless, these two studies clearly demonstrated the in vivo antidiabetic and anti-weight gaining efficacy of the extract.
Corosolic Acid and Lagerstroemin—The Most Effective Antidiabetic Compounds in Banaba Extract?
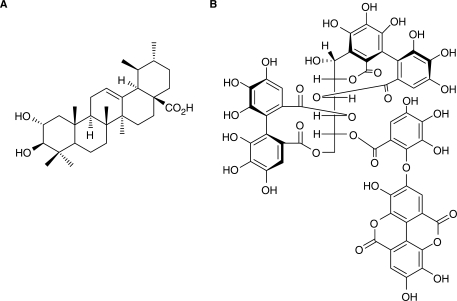
In 1993, a group of scientists from Hiroshima University used an Ehrlich ascites tumor cell line coupled with a bioassay guided fractionation to screen compounds isolated by HPLC from banaba extract in order to identify the effective antidiabetic component (21). Corosolic acid (2α-hydroxyursoloic acid) was identified as the effective compound in the methanol extract of banaba leaves in a glucose uptake assay (Fig. 2A, 21). However, this result should be considered with caution since the tumor cell line used in this study is a very unusual and unconventional cell line for diabetes studies or antidiabetic compound screening. Furthermore, the result could not explain the discrepancy that both the banaba water extract and the methanol extract were active in antidiabetic and anti-obesity animal studies since corosolic acid only exists in the methanol extract (19–21).
Figure 2.
Chemical structures of corosolic acid and Lagerstroemin. Corosolic acid (A) is a triterpenoid (21), not a tannin. Lagerstroemin (B) is an ellagitannin (22).
Realizing the potential problems associated with the cell line selection and assay method, the researchers acknowledged in a later publication that corosolic acid ‘could not represent the whole activity of the banaba extract’ (22). Consequently, the researchers switched their cell model from the original tumor cells to a natural cell target of insulin, adipocytes, in order to allow for the ‘isolation and the identification of more active compounds using the improved methodology’ (22). After HPLC purification, ellagitannins were identified in the water extract of banaba as the activators of glucose transport in fat cells with a glucose uptake assay (22). One of the most potent ellagitannins was named ‘Lagerstroemin’ (Fig. 2, 22).
In a recent study, the same group reported the activation of the insulin receptor (IR) by Lagerstroemin (Fig. 2, 23). In this study, Lagerstroemin was able to induce phosphorylation of the β-subunit of IR at 150 μM (23) but the mechanism responsible for IR activation was not found. The researchers speculated that Lagerstroemin could act intracellularly or bind to the insulin receptor (IR) extracellularly.
In 2004, a separate group of researchers from several universities found that glucose transporter 4 (GLUT4) translocation from the intracellular microsomal membrane to the plasma membrane was significantly increased in the muscle cells of mice treated orally with corosolic acid (P < 0.05, 24). This result is both interesting and puzzling. GLUT4 is the major glucose transporter protein in both muscle and adipocytes and GLUT4 is insulin-responsive (4,25). However, since corosolic acid does not possess insulin-like glucose transport stimulatory activity, the process that leads to GLUT4 translocation is not known. The GLUT4 membrane translocation mechanism initiated by corosolic acid as described (24) must be independent of the IR mediated signaling pathway since corosolic acid does not use this pathway for its activity. In 2006, the same group of researchers found that corosolic acid significantly reduces blood glucose levels and plasma insulin levels in KK-Ay diabetic mice (26). Additionally, this group of researchers published another article in 2006 in which they showed that corosolic acid significantly lowered blood glucose levels at the 90 min mark in an oral glucose tolerance test done on type 2 diabetic patients (27). However, no statistical difference between the treated group and the control group was found for any other time point during the test (27).
Tannic Acid and PGG—New Antidiabetic and Anti-adipogenic Polyphenolic Compounds
Our interest in the isolation and identification of antidiabetic compounds from natural sources initiated our investigation of banaba extracts with 3T3-L1 adipocytes as a cell model and a radioactive glucose uptake assay as a screening method for the identification of potential antidiabetic compounds. In our study, we confirmed that the water and methanol extracts of banaba leaves exhibit glucose transport stimulatory activity (28). In addition, we showed that the activity induced by the extract had a concentration-activity profile similar to that of insulin, suggesting that the activity of banaba extract (BE) might be triggered via an insulin-like mechanism (28). Furthermore, we demonstrated that the same BE also inhibits adipocyte differentiation (28). 3T3-L1 preadipocytes treated with methylisobutylxanthine, dexamethasone, and insulin (MDI) and BE did not differentiate into adipocytes as they normally do under the sole influence of MDI. This result indicated that BE inhibits the adipocyte differentiation activity induced by MDI. Unlike the glucose transport stimulation, the anti-differentiation activity is anti-insulin like, since insulin plays an important role in adipocyte differentiation and is a component of MDI (‘I’ in MDI stands for Insulin). Thus, BE differs from insulin in that it is anti-adipogenic whereas insulin is adipogenic, which is considered to be a negative side effect of insulin.
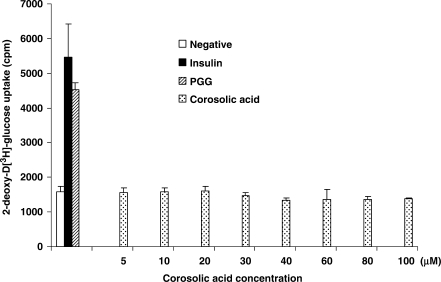
In order to identify the component(s) in BE responsible for the antidiabetic activity, our first goal was to confirm that corosolic acid was responsible for the glucose transport stimulatory activity. Since tannins comprise up to 40% of the material in the extract, we were interested in separating it from corosolic acid first. To our surprise, after removing tannins by either gelatin or bovine serum albumin tannin precipitations (29), the remaining extract was not able to induce glucose transport. We concluded that the glucose transport activity was caused by the tannin component of the extract, and not corosolic acid (29). Furthermore, we tested pure corosolic acid and found that it was not able to stimulate glucose transport in our cell model (Fig. 3). Although we cannot exclude the possibility that corosolic acid may have some antidiabetic activity, we can eliminate corosolic acid from having the insulin-like glucose transport stimulatory activity found in adipocytes. It should be noted that banaba extracts prepared from banaba leaves from different sources may have different chemical compositions, which in turn may lead to different experimental results (our lab used dried banaba leaves from the Philippines).
Figure 3.
Absence of glucose transport stimulatory activity of corosolic acid in adipocytes. Pure corosolic acid in aqueous solution was added to 3T3-L1 adipocytes grown in wells of 6-well cell culture plates to induce glucose transport by a commonly used procedure (28,29). Cells treated with either 1 μM insulin or 30 μM penta-galloyl-glucose (PGG) were used as positive controls. Cells treated with water vehicle served as negative controls. Samples were in triplicate in the experiment, and the experiment was repeated three times. No difference was found by a one-way ANOVA statistical analysis between the negative (vehicle) control and corosolic acid samples at any concentration.
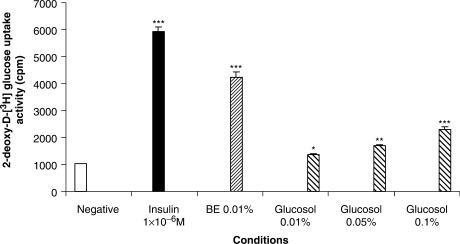
After testing corosolic acid, we also tested the corosolic acid-based banaba extract product glucosol (30). The glucose uptake assay revealed that glucosol was activating glucose transport. Its activity was dose-dependent (Fig. 4). However, since corosolic acid itself does not have any insulin receptor mediated glucose transport activity, the effect of glucosol is likely to originate from other chemical compounds in the banaba extract and not from corosolic acid.
Figure 4.
Glucose transport stimulatory activity of glucosol as compared with banana extract. Banaba extract (BE) was prepared in house (28). Glucosol (30) was purchased. Glucosol was compared with BE in a regular glucose uptake assay (28,29). Samples were assayed in triplicates, and the assay was repeated three times. The result of the assays was analyzed with a one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001. All samples were compared with the negative (vehicle) control.
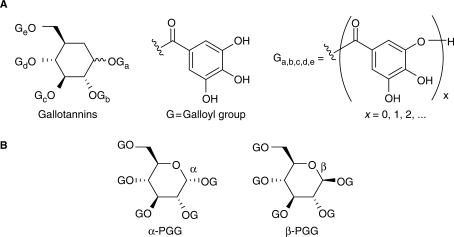
Tannins comprise a large and diverse class of polyphenolic compounds (31,32). Our search for the active components in the tannin fraction of BE was made much easier after we discovered that commercially available tannic acid (TA) shows similar glucose transport stimulatory activity to BE (29). The main components of TA are gallotannins, a subclass of the tannins usually consisting of a glucose core connected to a variable number of galloyl groups via ester bonds (Fig. 5).
Figure 5.
Chemical structures of gallotannins (tannic acid, A) and PGG (B). PGG exist in two anomeric forms.
TA is known to exhibit various health-beneficial activities (33–36). As a constituent of red wine, it has been shown to effectively reduce blood glucose levels in type 2 diabetes patients (37) and the production of endothelin-1 (38), a key protein factor intimately involved in the development of cardiovascular disease (38). In our study, TA was found to be much more potent and efficacious than the ellagitannin Lagerstroemin (29). Therefore, TA was chosen as the focus of our research for isolating active compounds from the tannin fraction of BE. Components of TA were separated by HPLC, and active fractions were identified with a glucose uptake assay in 3T3-L1 adipocytes (29). The study led to the discovery of penta-O-galloyl-D-glucopyranose (PGG) as the most effective compound in TA (Fig. 5, 39). Both anomers of PGG occur in nature (40). The α-anomer was found to be slightly more active than the β-form in its glucose transport stimulatory activity (39).
Corosolic Acid and Lagerstroemin versus Tannic Acid and PGG: Which are More Bioactive?
In the ellagitannin study mentioned earlier, Lagerstroemin exhibits glucose transport stimulation at 40 μM with an EC50 of 80 μM (22). In comparison, α- and β-PGG exhibit activity at a concentration as low as 10 μM with EC50 of 17 and 18 μM (39). In other words, α- and β-PGG are about five times more potent than Lagerstroemin in stimulating glucose transport. We would like to emphasize that both α- and β-PGG possess the adipogenesis inhibitory activity exhibited by the banaba extract (28) and by TA (29). This suggests that these two activities are associated with the same molecules. It also indicates the functional differences between insulin (glucose transport inducing and adipogenic) and PGG (glucose transport inducing but anti-adipogenic). Both compounds may have the potential to reduce hyperglycemia without increasing adiposity, a very desirable combination of properties that insulin lacks.
The Lagerstroemin study also showed that its glucose transport inducing activity is about 54% of that of insulin (22). In comparison, α- and β-PGG showed ∼60–70% of insulin's glucose transport inducing activity (39). Thus, α- and β-PGG are at least 30% more effective than Lagerstroemin (Table 1). It is interesting that PGG possesses many other health-beneficial bioactivities, such as anticancer (41,42), anti-inflammation (43,44), anti-virus (anti-HIV, 45; anti-SARS, 46) and antioxidant activity (47,48).
Table 1.
Comparison of potency and efficacy of the glucose transport stimulatory activity of different compounds isolated from Lagerstroemia speciosa
| Compound | Glucose transport activity (EC50 = potency) | Relative transport activity | Reference |
|---|---|---|---|
| Tannin-based BE | + | ∼70 | (28) |
| β-PGG (MW = 940) | 18 μM | ∼100* | (39) |
| Lagerstroemin (MW > 1200) | 80 μM | ∼70 | (22) |
| Corosolic acid | – | 0 | (21) |
| Insulin | ∼1 nM | 156 |
*Activity of β-PGG was arbitrarily assigned as 100.
Conclusion—Unfinished Story
From the known studies, we conclude that tannin molecules are responsible for the insulin-like glucose transport stimulatory activity of the banaba extract. Gallotannins such as PGG appear to be more potent and efficacious than ellagitanins such as Lagerstroemin in IR binding, IR activation and glucose transport induction. Corosolic acid does not possess any insulin-like glucose transport stimulatory activity. If its antidiabetic activity can be confirmed, it is likely to be induced via a non-insulin like, indirect mechanism.
Although tannins were identified as the effective component for the insulin like glucose transport inducing activity in banaba extract, the most effective tannin molecule in the extract has not been identified. A well designed bioassay (glucose uptake assay) guided isolation should be able to complete this task.
In the past 10 years, other studies regarding banaba extracts or chemicals derived from banaba extracts were reported (49–52). These studies indicate that banaba extracts contain interesting biomedical substances that have attracted significant scientific attention. More detailed studies at molecular and cellular levels as well as in animal models are required to elucidate banaba extract's antidiabetic activity and other health beneficial activities such as its anti-adipogenesis activity.
Acknowledgments
We would like to thank Dr Jay Shubrook for his review of the manuscript.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Astrup A, Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev. 2000;2:57–9. doi: 10.1046/j.1467-789x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 5.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 6.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Moller DE. New approaches in the treatment of type 2 diabetes. Curr Opin Chem Biol. 2000;4:461–7. doi: 10.1016/s1367-5931(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 8.Samane S, Noel J, Charrouf Z, Amarouch A, Haddad PS. Insulin-sensitizing and anti-proliferative effects of Argania spinosa seed extracts. Evid Based Complement Alternat Med. 2006;3(3):317–27. doi: 10.1093/ecam/nel015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo JZ, Luo L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured β cells. Evid Based Complement Alternat Med. 2006;3(3):365–72. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punitha IS, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2(3):375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Gosha-jinki-gan (a herbal complex) corrects abnormal insulin signaling. Evid Based Complement Alternat Med. 2004;1(3):269–76. doi: 10.1093/ecam/neh028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;2(4):513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu JH, Wu YC, Liou SS, Liu IM, Huang LW, Cheng JT. Mediation of endogenous beta-endorphin by tetrandrine to lower plasma glucose in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2004;1(2):193–201. doi: 10.1093/ecam/neh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia F. On the hypoglycemic effect of decoction of Lagerstroemia speciosa leaves (banaba) administered orally. J Phil Med Assoc. 1940;20:395–402. [Google Scholar]
- 15.Garcia F. Distribution and deterioration of insulin-like principle in Lagerstroemia speciosa (banaba) Acta Med Philippina. 1941;3:99–104. [Google Scholar]
- 16.Garcia F. Plantisul compared with insulin. J Phil Med Assoc. 1955;31(6):276–82. [PubMed] [Google Scholar]
- 17.Garcia F. Plantisul tablets in the treatment of diabetes mellitus. J Phil Med Assoc. 1956;31:216–24. [PubMed] [Google Scholar]
- 18.Garcia F, Melencio-Maglalang P. Application of banabins (a plantisul preparation) and S.B. menus to diabetics. J Phil Med Assoc. 1957;33(1):7–15. [PubMed] [Google Scholar]
- 19.Kakuda T, Sakane I, Takihara T, Ozaki Y, Takeuchi H, Kuroyanagi M. Hypoglycemic effect of extracts from Lagerstroemia speciosa L. leaves in genetically diabetic KK-AY mice. Biosci Biotechnol Biochem. 1996;60(2):204–8. doi: 10.1271/bbb.60.204. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Unno T, Ushitani M, Hayashi K, Kakuda T. Antiobesity activity of extracts from Lagerstroemia speciosa L. leaves on female KK-Ay mice. J Nutr Sci Vitaminol. 1999;45(6):791–5. doi: 10.3177/jnsv.45.791. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 21.Murakami C, Myoga K, Kasai R, Ohtani K, Kurokawa T, Ishibashi S, et al. Screening of plant constituents for effect on glucose transport activity in Ehrlich Ascites tumor cells. Chem Pharm Bull. 1993;41(12):2129–31. doi: 10.1248/cpb.41.2129. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Maruyama H, Kasai R, Hattori K, Takasuga S, Hazeki O, et al. Ellagitannins from Lagerstroemia speciosa as activators of glucose transport in fat cells. Planta Med. 2002;68(2):173–5. doi: 10.1055/s-2002-20251. [DOI] [PubMed] [Google Scholar]
- 23.Hattori K, Sukenobu N, Sasaki T, Takasuga S, Hayashi T, Kasai R, et al. Activation of insulin receptors by lagerstroemin. J Pharmacol Sci. 2003;93(1):69–73. doi: 10.1254/jphs.93.69. [DOI] [PubMed] [Google Scholar]
- 24.Miura T, Itoh Y, Kaneko T, Ueda N, Ishida T, Fukushima M, et al. Corosolic acid induces GLUT4 translocation in genetically type 2 diabetic mice. Biol Pharm Bull. 2004;27(7):1103–5. doi: 10.1248/bpb.27.1103. [DOI] [PubMed] [Google Scholar]
- 25.Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- 26.Miura T, Ueda N, Yamada K, Fukushima M, Ishida T, Kaneko T, et al. Antidiabetic effects of corosolic acid in KK-Ay diabetic mice. Biol Pharm Bull. 2006;29(3):585–7. doi: 10.1248/bpb.29.585. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima M, Matsuyama F, Ueda N, Egawa K, Takemoto J, Kajimoto Y, et al. Effect of corosolic acid on postchallenge plasma glucose levels. Diabetes Res Clin Pract. 2006;73:174–7. doi: 10.1016/j.diabres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Kim J, Li Y, Liu X, Li J, Chen X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J Nutr. 2001;131(9):2242–7. doi: 10.1093/jn/131.9.2242. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Kim JK, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J Nutr. 2005;135(2):165–71. doi: 10.1093/jn/135.2.165. [DOI] [PubMed] [Google Scholar]
- 30.Judy WV, Hari SP, Stogsdill WW, Judy JS, Naguib YM, Passwater R. Antidiabetic activity of a standardized extract (Glucosol) from Lagerstroemia speciosa leaves in type II diabetics. A dose-dependence study. J Ethnopharmacol. 2003;87(1):115–7. doi: 10.1016/s0378-8741(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 31.Haslam E. Plant Polyphenols: Vegetable Tannins Revisited. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- 32.Waterman PG, Mole S. Analysis of Phenolic Plant Metabolites. Oxford: Blackwell Scientific Publications; 1994. [Google Scholar]
- 33.Huang MT, Osawa T, Rosen RT, Ho CT, editors. Food Phytochemicals for Cancer Prevention I: Fruits and Vegetables. Washington DC: American Chemical Society; 1994. [Google Scholar]
- 34.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–117. [Google Scholar]
- 35.Hagerman AE, Riedl KM, Jones JA, Sovik KN, Ritchard NT, Hartzfeld PW, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–92. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 36.Feldman KS, Sahasrabudhe K, Lawlor MD, Wilson SL, Lang CH, Scheuchenzuber WJ. In vitro and in vivo inhibition of LPS-stimulated tumor necrosis factor-alpha secretion by the gallotannin beta-D-pentagalloylglucose. Bioorg Med Chem Lett. 2001;11:1813–5. doi: 10.1016/s0960-894x(01)00332-8. [DOI] [PubMed] [Google Scholar]
- 37.Gin H, Rigalleau V, Caubet O, Masquelier J, Aubertin J. Effects of red wine, tannic acid, or ethanol on glucose tolerance in non-insulin-dependent diabetic patients and on starch digestibility in vitro. Metabolism. 1999;48:1179–83. doi: 10.1016/s0026-0495(99)90135-x. [DOI] [PubMed] [Google Scholar]
- 38.Corder R, Douthwaite JA, Lees DM, Khan NQ, Santos ACV, Wood EG, et al. Endothelin-1 synthesis reduced by red wine. Nature. 2001;414:863–4. doi: 10.1038/414863a. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Kim J, Li J, Liu F, Liu X, Himmeldirk K, et al. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-d-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem Biophys Res Commun. 2005;336(2):430–7. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 40.Nishizawa M, Yamagishi T, Nonaka G, Nishioka I, Bando H. Novel hydrolyzable tannins from Nuphar Japonicum DC. Chem Pharm Bull. 1982;30:1094–7. [Google Scholar]
- 41.Lee SH, Ryu SY, Choi SU, Lee CO, No Z, Kim SK, et al. Hydrolysable tannins and related compound having cytotoxic activity from the fruits of Terminalia chebula. Arch Pharm Res. 1995;18:118–20. [Google Scholar]
- 42.Lee SH, Park JS, Kim SY, Chung SR, Choi SU. Cytotoxic effects of hydrolysable tannins from some euphorbia plants on the human tumour cell lines. Yakhak Hoechi. 1997;41:524–9. [Google Scholar]
- 43.Feldman KS, Sahasrabudhe K, Smith RS, Scheuchenzuber WJ. Immunostimulation by plant polyphenols: a relationship between tumour necrosis factor-α production and tannin structure. Bioorg Med Chem Lett. 1999;9:985–90. doi: 10.1016/s0960-894x(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 44.Feldman KS, Sahasrabudhe K, Laulor MD, Wilson SL, Lang CH, Scheuchenzuber WJ. In vitro and in vivo inhibition of LPS- stimulated tumour necrosis factor-α secretion by the gallotannin β-D-pentagalloylglucose. Bioorg Med Chem Lett. 2001;11:1813–5. doi: 10.1016/s0960-894x(01)00332-8. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima H, Ichiyama K, Hirayama F, Uthino K, Ito M, Saitoh T, et al. Sulfated penta galloylglucose (Y-ART-3) inhibits HIV replication and cytopathic effects in vitro, and reduces HIV infection in hu-PBL-SCID mice. Antivi Res. 1996;30:95–108. doi: 10.1016/0166-3542(95)00903-5. [DOI] [PubMed] [Google Scholar]
- 46.Chen CN, Lin CP, Huang KK, Chen WC, Hsieh HP, Liang PH, Hsu JT. Inhibition of SARS-CoV 3C-like protease activity by Theaflavin-3,3′-digallate (TF3) Evid Based Complement Alternat Med. 2005;2(2):209–15. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka N, Nishikawa K, Ishimaru K. Antioxidative capacity of extracts and constituents in Cornus capitata adventitious roots. J Agri Food Chem. 2003;51:5906–10. doi: 10.1021/jf030267s. [DOI] [PubMed] [Google Scholar]
- 48.Okubo T, Nagai F, Seto T, Satoh K, Ushiyama K, Kano I. The inhibition of phenylhydroguinone-induced oxidative DNA cleavage by constituents of Moutan cortex and Paeoniae radix. Biol Pharm Bull. 2000;23:199–203. doi: 10.1248/bpb.23.199. [DOI] [PubMed] [Google Scholar]
- 49.Hong H, Jai Maeng W. Effects of malted barley extract and banaba extract on blood glucose levels in genetically diabetic mice. J Med Food. 2004;7(4):487–90. doi: 10.1089/jmf.2004.7.487. [DOI] [PubMed] [Google Scholar]
- 50.Matsuura T, Yoshikawa Y, Masui H, Sano M. Suppression of glucose absorption by various health teas in rats. Yakugaku Zasshi. 2004;124(4):217–23. doi: 10.1248/yakushi.124.217. [Japanese] [DOI] [PubMed] [Google Scholar]
- 51.Hosoyama H, Sugimoto A, Suzuki Y, Sakane I, Kakuda T. Isolation and quantitative analysis of the alpha-amylase inhibitor in Lagerstroemia speciosa (L.) Pers. (Banaba) Yakugaku Zasshi. 2003;123(7):599–605. doi: 10.1248/yakushi.123.599. [Japanese] [DOI] [PubMed] [Google Scholar]
- 52.Park MY, Lee KS, Sung MK. Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-α, PPAR-γ and LPL mRNA expressions. Life Sci. 2005;77:3344–54. doi: 10.1016/j.lfs.2005.05.043. [DOI] [PubMed] [Google Scholar]