Abstract
Chlorophytum borivilianum Santapau & Fernandes (Liliaceae) is a very popular herb in traditional Indian medicine and constitute a group of herbs used as ‘Rasayan’ or adaptogen. Ethanolic extract of the roots and its sapogenin were evaluated for their immunomodulatory activity. Effect of azathioprine-induced myelosuppresion and administration of extracts on hematological and serological parameters was determined. Administration of extracts greatly improved survival against Candida albicans infection. An increase in delayed-type hypersensitivity response (DTH), % neutrophil adhesion and in vivo phagocytosis by carbon clearance method was observed after treatment with extracts. Immunostimulant activity of ethanolic extract was more pronounced as compared to sapogenins. The results, thus justifies the traditional use of C. borivilianum as a rasayana drug.
Keywords: Chlorophytum borivilianum, immunomodulator, rasayana, Safed Musli
Introduction
Scientific literature is continuously reporting plant drugs having immunomodulatory activity. Most of the leads for this activity are from traditional medicines from different parts of the world (1,2). The Indian system of medicine ‘Ayurveda’, conceptualizes a category of drug activity known as ‘Rasayana’. The word Rasayana is composed of two words ‘Rasa’ meaning elixir and ‘Ayana’ meaning house. The word therefore signifies property of the plant that helps to rejuvenate the system, i.e. adaptogenic activity (3). ‘Rasayan’ therapy prevents diseases and counteracts the aging process by means of optimization or homeostasis. Many plants have been extensively used as ‘Rasayana’ drugs in Ayurveda for the management of neurodegenerative diseases, as rejuvenators, immunomodulators, aphrodisiac and nutritional supplements (4–7).
Safed Musli has been described in ancient Indian literature such as Bhavaprakash nighantu, Rasendra Sarsangrah, Raja Ballabh Nighantu as `Vajikaran' or aphrodisiac which is a special type of immunomodulator (8–10). Safed Musli is a controversial drug and various herbs are employed as Safed Musli by practitioners of Indian system of medicine. One of the popular and marketed herb under this nomenclature is Chlorophytum borivilianum Santapau & Fernandes (Liliaceae) (11,12).
Since the roots of C. borivilianum are employed as ‘Rasayana’ (13,14) and also because Safed Musli is a constituent of ‘Chyawanprash’, an outstanding rejuvenator (8). It was considered worthwhile to investigate this drug for immunomodulatory activity.
Materials and Methods
Plant Material
C. borivilianum roots were obtained from Jeevan Agro farms (Sagar, Madhya Pradesh, India). A herbarium of the source was made and identified. A voucher specimen no. CB-MT16 has been submitted at the departmental herbarium of Department of Pharmaceutical Sciences Dr H.S. Gour Vishwavidyalaya, Sagar (MP, India). The roots were dried in sunlight and coarsely powdered for extraction.
Extraction and Fractionation
Powdered roots were defatted with petroleum ether (60–80°C). Ethanolic extract of the drug was prepared by extracting defatted roots with 95% ethanol in Soxhlet extractor (yield 16% w/w). For isolation of sapogenin, ethanolic extract as obtained above was suspended in water (100 ml). It was then extracted with n-butanol (300 ml), the volume of n-butanol soluble portion was then reduced to half under reduced pressure and finally saponins were precipitated by addition of diethyl ether. The collected precipitate was hydrolyzed using hydrochloric acid (2 N HCl) and the precipitated sapogenins were collected (15).
Alternatively, 500 g defatted drug powder was hydrolyzed by using 200 ml of 4 N H2SO4 in a round bottom flask which was kept overnight at room temperature and filtered to remove acidic solution, marc was washed three to four times with cold distilled water. After washing and drying the marc was refluxed for 30 min using fresh 200 ml of 4 N H2SO4 to ensure complete hydrolysis of drug. The mixture was filtered and filtrate was discarded. Hydrolyzed drug powder was further washed with distilled water three times to ensure complete removal of acid. The hydrolyzed drug powder was dried in an air oven to ensure completely dried drug powder. The hydrolyzed drug powder was fed in a Soxhlet extractor and subjected to extraction with ethanol (95%). Ethanol was removed from the extract under reduced pressure and sapogenins were collected (16). The sapogenins isolated by this method were used for pharmacological studies.
Sapogenins isolated by either method exhibited identical TLC profile suggesting suitability of both the process for isolation of sapogenins from the drug. HPTLC analysis of the ethanolic extract as well as isolated sapogenin fraction was performed. In brief, the chromatographic analysis of ethanolic extract was performed on silica gel-GF254 precoated plates (E Merck, Germany) using chloroform:glacial acetic acid: methanol:water (16:8:3:2, v/v) as mobile phase, 10 spots were visualized upon derivatization with anisaldehyde sulfuric acid reagent. Sapogenin fraction was characterized on chloroform: diethylether (1:1, v/v) mobile phase against β-sitosterol as standard marker. The pattern and standardization of the sapogenins against standard marker (β-sitosterol) has been reported by us previously (17).
Materials
Sheep red blood cells (SRBC's) were obtained from Haffkine Biopharmaceuticals Ltd, Mumbai, India, and were washed thrice with large volumes of pyrogen-free sterile saline and adjusted to a concentration of 5 × 109 cells per ml for immunization and challenge. Azathioprine was obtained as gift sample from Troika Pharmaceuticals, Ahmedabad, India. Candida albicans was purchased from IMTECH (Chandigarh, India).
Rats
Wistar strain albino rats weighing between 140–150 gm of either sex were used. They were housed in departmental animal room under standard condition of temperature (24 ± 1°C), 12/12 h light/dark cycle and fed on standard pellet diet. Ethanolic extract and sapogenin were administered orally as suspension in 2% polyvinyl pyrolidine solution using metal canula.
Toxicity studies were performed and dose of 1 gm kg−1 body weight (bw) of ethanolic extract or sapogenins did not cause any toxic effect.
Statistical Analysis
Data are expressed as mean ± SEM and analyzed for significance by Dunnet's test (comparing all versus control) using Instat v.2.02 software (Graphpad software Inc.) residing in Pentium IV processor run on Windows Xp.
Experimental
Treatment
Albino rats were divided into groups comprising of six animals each. Group I served as control and was administered vehicle only. Group II was administered 200 mg kg−1 bw ethanolic extract. Group III received 100 mg kg−1 bw sapogenin extract. Each experiment was performed on fresh group of animals unless specified.
Non-Specific Immunity Determined by Survival Rate Against Fungal Infection
Treatments of all the three groups began 14 days before challenge. On the day of challenge all groups were injected with 5 × 107 viable C. albicans cells and observed daily for mortality for a period of 10 days.
In Vivo Phagocytosis Using Carbon Clearance Method
The method of Biozzi et al. (1953) (18,19) was used. Treatments of all groups started 14 days before experimentation. On 15th day of treatment, mice were injected with 0.1 ml of carbon suspension (Pellikan Tuschea Ink, Germany) intravenously through tail vein. Blood samples (25 μl) were collected from retro-orbital plexus just before and at 4, 8, 12 and 16 min after injection. Blood samples were lyzed with 2 ml of 0.1% acetic acid and absorbance of samples recorded at 675 nm (20). The graph for absorbance versus time was plotted for each animal in respective test group and phagocytic index was calculated using the formula:
where Ksample represents the slope of absorbance versus time curve for extract-treated samples and Kstandard represents the slope of absorbance versus time curve for blood sample collected before treatment.
SRBC-Induced Delayed-Type Hypersensitivity Reaction (DTH Response)
The method of Lagrange et al. (1974) (21) was used. Treatments with extracts began 14 days before challenge. All the groups were immunized by injecting 20 μl of 5 × 109 SRBC per ml subcutaneously into the right foot pad. After 14 days of treatment the thickness of left foot pad was measured using calipers (Schnelltester, Germany) reading to 0.01 mm. The mice were then challenged by injecting 20 μl of 5 × 109 SRBC per ml intradermally on the left hind foot pad (time 0). Foot thickness was measured after +24 and +48 h of challenge. The difference between the thickness of left foot just before and after challenge in mm was taken as a measure of DTH (22).
Neutrophil Adhesion Test. The method described by Wilkonson (1978) (23) was used for evaluating the effect of extracts on neutrophil adhesion. After 14 days of treatment of all the three groups, blood samples were collected by retro-orbital puncture in heparinized vials and subjected to total as well as differential leukocyte count. After initial counts the blood samples were incubated with 80 mg ml−1 of nylon fibers at 37°C for 15 min. The incubated samples were again analyzed for total and differential leukocyte count. The product of total leukocyte count and % neutrophil known as neutrophil index was determined for each of the respective groups (24). The % neutrophil adhesion for each of the test groups was determined as follows,
 |
Activity Against Drug-Induced Immunosuppression
The methods of Doherty (1981) (25) modified by Ziauddin et al. (26) was used. Six groups of six albino mice each were taken. Group I served as control. Group II was administered ethanolic extract 200 mg kg−1 bw. Group III was given sapogenin extract 100 mg kg−1 bw. Group IV received 100 mg kg−1 bw azathioprine. In Group V animals, 100 mg kg−1 bw azathioprine and 200 mg kg−1 bw of ethanolic extract was administered, whereas Group VI was treated with 100 mg kg−1 bw azathioprine and 100 mg kg−1 bw of sapogenin extract. All the animals were sensitized by injecting 5 × 109 SRBC's intraperitoneally before experimentation.
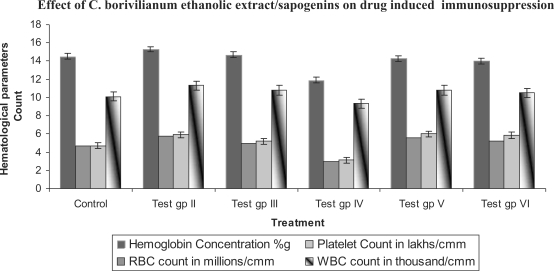
On 15th of treatment, all the mice were sacrificed and blood was collected in heparinized vials. Blood samples for animals of each group were subjected for hematological and serological studies such as hemoglobin content, total RBC, total and differential WBC count and platelet count (Fig. 1 and Table 2).
Figure 1.
Effect of Chlorophytum borivillianum Sant. & F. on azathioprine induced suppression of hematological parameters.
Table 2.
Effect of administration of C. borivilianum ethanolic extract/sapogenins on non-specific and specific immune systems
| Groups | Haemoglobin concentration (g%) | Platelet count in thousand per cmm | RBC count in millions per cmm | WBC count in thousand per cmm |
|---|---|---|---|---|
| Test Group I | 14.5 ± 0.31 | 468.5 ± 0.51 | 4.7 ± 0.31 | 10.1 ± 0.38 |
| Test Group II | 15.3 ± 0.25b | 578.5 ± 0.56c | 5.9 ± 0.57a | 11.3 ± 0.47a |
| Test Group III | 14.7 ± 0.48a | 496.6 ± 0.56 | 5.2 ± 0.12 | 10.8 ± 0.28a |
| Test Group IV | 11.9 ± 0.32c | 300.6 ± 0.24c | 3.13 ± 0.43b | 9.33 ± 0.45a |
| Test Group V | 14.3 ± 0.11a | 560.45 ± 0.42c | 5.99 ± 0.35b | 10.8 ± 0.38a |
| Test Group VI | 14.0 ± 0.09a | 524.65 ± 0.39b | 5.86 ± 0.31b | 10.5 ± 0.46a |
Test Group I: control (no treatment); Test Group II: ethanolic extract 200 mg per kg b.w; Test Group III: sapogenin extract 100 mg per kg b.w; Test Group IV: azathioprine 100 mg per kg b.w; Test Group V: azathioprine 100 mg per kg b.w; and ethanolic extract 200 mg per kg b.w; Test Group VI: azathioprine 100 mg per kg b.w. and Sapogenin extract 100 mg per kg b.w.
aP > 0.5 not significant.
bP < 0.05 significant.
cP < 0.005 extremely significant.
Results
Survival Rate Studies
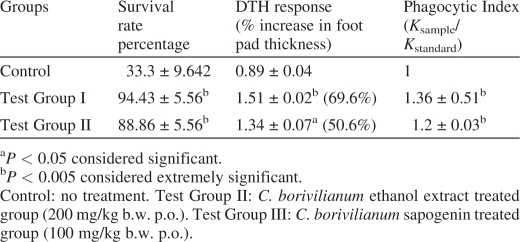
The survival rate of the treated animals was considerably enhanced after treatment with C. borivilianum extract. Administration of ethanolic extract and sapogenin exhibited 94 ± 5.56 and 88.86 ± 5.6 percent survival after infection with C. albicans. Sapogenin extract was 50% (P < 0.05) and ethanolic extract 60 % (P < 0.05) more effective in reducing mortality as compared to control group animals (Table 1). The results suggest potentiation of non-specific immune response on treatment with ethanolic extract and sapogenins.
Table 1.
Effect of administration of C. borivilianum ethanolic extract/ sapogenins on non-specific and specific immune systems
 |
DTH Using SRBC as an Antigen
In the control group animals, after +48 and +72 h of challenge the DTH response was either equal or slightly more than the 0 h response; therefore, the peak edema after +24 h of challenge was the evaluating parameter. Ethanolic extract (200 mg kg−1 per orally) was most effective (P < 0.05) compared to sapogenin (100 mg kg−1 per orally) treatment in increasing the delayed-type hypersensitivity response (Table 1).
Rate of Carbon Clearance
Rate of carbon clearance is the measure of competency of the reticuloendothelial system and its granulopoetic activity (14), the faster removal of carbon particles has been correlated with the enhanced phagocytic activity. In the present study an increased phagocytic activity was observed in treated groups as compared to control. The rate of carbon clearance which was determined as phagocytic index was ∼20 % (1.2 ± 0.03) and ∼25% (1.36 ± 0.51) greater in sapogenins and ethanolic extract-treated groups, respectively, clearly indicating an enhanced in vivo phagocytic activity (Table 1).
Effectiveness Against Drug-Induced Immunosuppression
Administration of ethanolic extracts and sapogenins in general had a beneficial effect on hematological profile wherein all the parameters such as hemoglobin, platelets, RBC and WBC counts were increased. Simultaneous administration of ethanolic extract and sapogenins along with azathioprine resulted in restoration of suppressed values observed after azathioprine treatment alone. Platelet, hemoglobin, RBC and WBC values observed were better than untreated control groups. Among the two test drugs ethanolic extract was pronouncedly more effective (P < 0.005) followed by sapogenin treatment (P < 0.05) (Fig. 1 and Table 2).
Neutrophil Adhesion Test
This test is an indicative of the marginalization of phagocytic cells in the blood vessels, i.e. an indication of immunostimulation. The % neutrophil adhesion in control group animals was 23.34 ± 2.1, in ethanolic extract-treated group it was 27.21 ± 1.6 whilst for sapogenin extract-treated group it was 25.62 ± 1.4. As is evident from the results of neutrophil adhesion test, nearly 10% (P < 0.5) increase in neutrophil adhesion is observed after administration of sapogenin extract, whilst a significant 17% (P < 0.05) increase in neutrophil adhesion is observed in ethanolic extract (Table 3).
Table 3.
Effect of C. borivilianum ethanolic extract/sapogenins on neutrophil adhesion in rats (mean ± SE)
| Animal group | TLC (103 mm−3) (X) | % Neutrophil (Y) | Neutrophil Index (X Y) | % Neutrophil adhesion | |||
|---|---|---|---|---|---|---|---|
| Un B | FTB | Un B | FTB | Un B | FTB | ||
| Group I | 6.4 ± 2.8 | 5.9 ± 2.2 | 44 ± 2.23 | 37 ± 3.2 | 284.8 ± 35.1 | 218.3 ± 25.4 | 23.34 ± 2.1 |
| Group II | 6.9 ± 1.4 | 6.1 ± 1.6 | 52 ± 2.21 | 43 ± 1.38 | 362.2 ± 32.4 | 265.1 ± 31.2 | 27.21 ± 1.6b |
| Group III | 6.8 ± 1.2 | 6.1 ± 1.4 | 51 ± 2.23 | 44 ± 1.47 | 356.6 ± 31.4 | 268.8 ± 32.1 | 25.62 ± 1.4a |
UnB: untreated blood; FTB: nylon fiber treated blood; Group I: control (no treatment); Group II: C. borivilianum ethanolic extract 200 mg per kg b.w; Group III: C. borivilianum sapogenin extract 200 mg per kg b.w.
aP < 0. 5 not significant.
bP < 0.05 considered significant.
Discussion
The scientific evidences collected from the study supports the traditional claims behind usage of the herb C. borivilianum, which is being cultivated and marketed extensively in India and abroad for medicinal purposes (12). The study affirms that C. borivilianum root extract is an effective immunostimulatory principle. The inference that can be drawn from the present study is that the total ethanolic extract is superior over sapogenin fraction of the plant as far as immunostimulatory activity is concerned. The extract does not only potentiate non-specific immune response, but is also effective in improving humoral as well as cell-mediated immunity. The effectiveness of extract-treated animals in overcoming the side effects of drug-induced immunosuppression provides evidence for balancing and adaptogenic effectiveness of extracts.
Use of herbs for improving the overall resistance of body against common infections and pathogens has been a guiding principle of Ayurveda (27,28). Chlorophytum spp. have been used and reported in many such formulations. The increase in survival rate is a general marker exhibiting potency of the ethanolic extract to overcome infectious condition. Increased carbon clearance is an indicator of enhanced in vivo phagocytic activity and competency of granulopoetic system in removal of foreign particle, thereby an indicator of enhanced immunological response against foreign particles or antigens. Increase in percent neutrophil adhesion is attributed due to marginalization of phagocytic cells, i.e. improved defensive response under normal circumstances. This study, apart from confirming the immunostimulant activity of C. borivilianum also, presents evidence for the presence of the substance other than sapogenins which induce stimulation of immune response in treated animals. Therefore, the plant holds promise for being used as an immunostimulating agent and an in-depth study on various fractions of the extract effective as immunomodulating entities from the plant is warranted to determine the most potent immunostimulating fraction from C. borivilianum. Thus, the study validates the traditional use of herb as a ‘Rasayana’ in Ayurvedic system of medicine (23).
Acknowledgments
The authors are grateful to Troika Pharmaceuticals (Ahmedabad, India) for providing gift sample of Azathioprine and to Jeevan Agro farms Pvt. Ltd for providing C. borivilianum roots. One of the authors Mayank Thakur is also thankful to University Grants Commission for providing financial support.
REFERENCES
- 1.Mukherjee PK. Phytopharmacology in the evaluation of herbal drugs. J Pharma Res. 2002;1:45–54. [Google Scholar]
- 2.Gauniyal AK, Rawat AKS, Pushpangadan P. Interactive Meeting for Evidenced-Based Complementary and Alternative Medicines: a Report. Evid Based Complement Alternat Med. 2005;2:249–52. [Google Scholar]
- 3.Handa SS. Rasayana drugs. In: Handa SS, Kaul MK, editors. Supplement to Cultivation and Utilization of Medicinal Plants. Vol. 1. Jammu Tawi: Regional Research Laboratory; 1996. pp. 509–10. [Google Scholar]
- 4.Tandon M, Shukla YN, Thakur RS. 4 Hydroxy, 8-11 oxidoheneicosol and other constituent for Chlorophytum arundinaceum roots. Phytochemistry. 1992;3:2525–8. [Google Scholar]
- 5.Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease management and the role of ‘Rasayana’ herbs of Ayurveda. J Ethnopharmacol. 2005;99:165–78. doi: 10.1016/j.jep.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Thakur M, Dixit VK. Effect of Chlorophytum borivilianum on androgenic and sexual behavior of male rats. Indian Drugs. 2006;43:300–6. [Google Scholar]
- 7.Joshi H, Parle M. Brahmi Rasayana improves learning and memory in mice. Evid Based Complement Alternat Med. 2006;3:79–85. doi: 10.1093/ecam/nek014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triveni A. Rasendrasarasangrah: Vajikaranadhikar. Rajkot, India. Nutan Press; 1976. pp. 617–43. [Google Scholar]
- 9.Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd edition. Allahabad, India: Lalit Mohan Basu; 1956. pp. 235–46. [Google Scholar]
- 10.Sharma SK, Chunekar KC, Paudal K. New Delhi: RAV publications Director Rashtriya Ayurveda Vidyapeeth; Plants of Sharangdhar Samhita; pp. 221–2. [Google Scholar]
- 11.Tandon M, Shukla YN. Phytoconstituents of Asparagus adescendens, Chlorophytum arundinaceum and Curculigo orichoides: a review. CROMAP. 1995;12:202–4. [Google Scholar]
- 12.Kothari SK. Safed Musli (Chlorophytum borivilianum) revisited. J Med Arom Plant Sci. 2004;26:60–3. [Google Scholar]
- 13.Puri HS. Rasayana—Ayurvedic Herbs for Longevity and Rejuvenation. London: Taylor and Francis; 2003. pp. 2003, 212–24. [Google Scholar]
- 14.Mishra SN. Bhaisajya Ratnavali. 1st edition. Varanasi: Chaukambha Surbharti Prakashan; 2005. pp. 2005, 1008–133. [Google Scholar]
- 15.Brain KR, Turner TD. The Practical Evaluation of Phytopharmaceuticals. Vol III. Bristol, UK. Wright-Scientechnica; 1975. pp. 104–44. [Google Scholar]
- 16.Paech K, Tracey MV. Modern Methods of Plant Drug Analysis. Vol. II. Gottingen: Springer Verlag; 1955. pp. 1995, 155–97. [Google Scholar]
- 17.Govindarajan R, Sreevidya N, Vijayakumar M, Thakur M, Dixit VK, Mehrotra S, et al. Standardization and determination of antioxidant activity of Chlorophytum borivilianum. Nat Prod Sci. 2005;11:165–9. [Google Scholar]
- 18.Biozzi G, Benacerraf B, Halpern BN. Quantitative study of the granulopoetic activity of reticulo endothelial system II: a study of kinetics of the RES relationship between weight of organs and their activity. Br J Exp Biol. 1953;34:441–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Halpern BN, Bancerraf B, Biozzi G. Quantitative study of the granulopoetic activity of the reticulo endothelial system I: the effect of the ingredients present in India ink and of substances affecting blood clotting in-vivo on the fate of carbon particles administered intravenously in rats, mice and rabbits. Br J Exp Biol. 1953;34:426–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Damre AS, Gokhale AB, Phadke AS, Kulkarni KR, Saraf MN. Studies on the immunomodulator activity of flavonoidal fraction of Tephrosia purpurea. Fitoterapia. 2003;74:257–61. doi: 10.1016/s0367-326x(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 21.Lagrange PH, Mackaness GB, Miller TE. Potential of T cell mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974;139:1529–39. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atal CK, Sharma ML, Kaul A, Khajuria A. Immunomodulatory agents of plant origin: I preliminary screening. J Ethnopharmacol. 1983;8:133–41. doi: 10.1016/0378-8741(86)90025-5. [DOI] [PubMed] [Google Scholar]
- 23.Wilkonson PC. Neutrophil adhesion test. In: Vane JR, Ferraria SH, editors. Handbook of Experimental Pharmacology. Vol. 1. Berlin: Springer Verlag; 1978. p. 1978, 109. [Google Scholar]
- 24.Fulzele SV, Burchandi PM, Kanoje VM, Joshi SB, Dorle AK. Immunostimulant activity of Asthamangal ghrita in rats. Ind J Pharmacol. 2002;34:194–7. [Google Scholar]
- 25.Doherty NS. Selective effects of immunosuppressive agents against the delayed hypersensitivity response and humoral response to sheep red blood cells in mice. Agents Actions. 1981;11:237–42. doi: 10.1007/BF01967620. [DOI] [PubMed] [Google Scholar]
- 26.Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B. Studies on immunomodulatory effects of Ashwagandha. J Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 27.The Wealth of India. Vol 1. New Delhi, India: National Institute of Science Communication, CSIR; 2002. Anonymous; p. , 247. [Google Scholar]
- 28.Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465–73. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]