Abstract
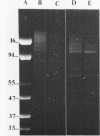
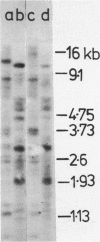
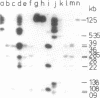
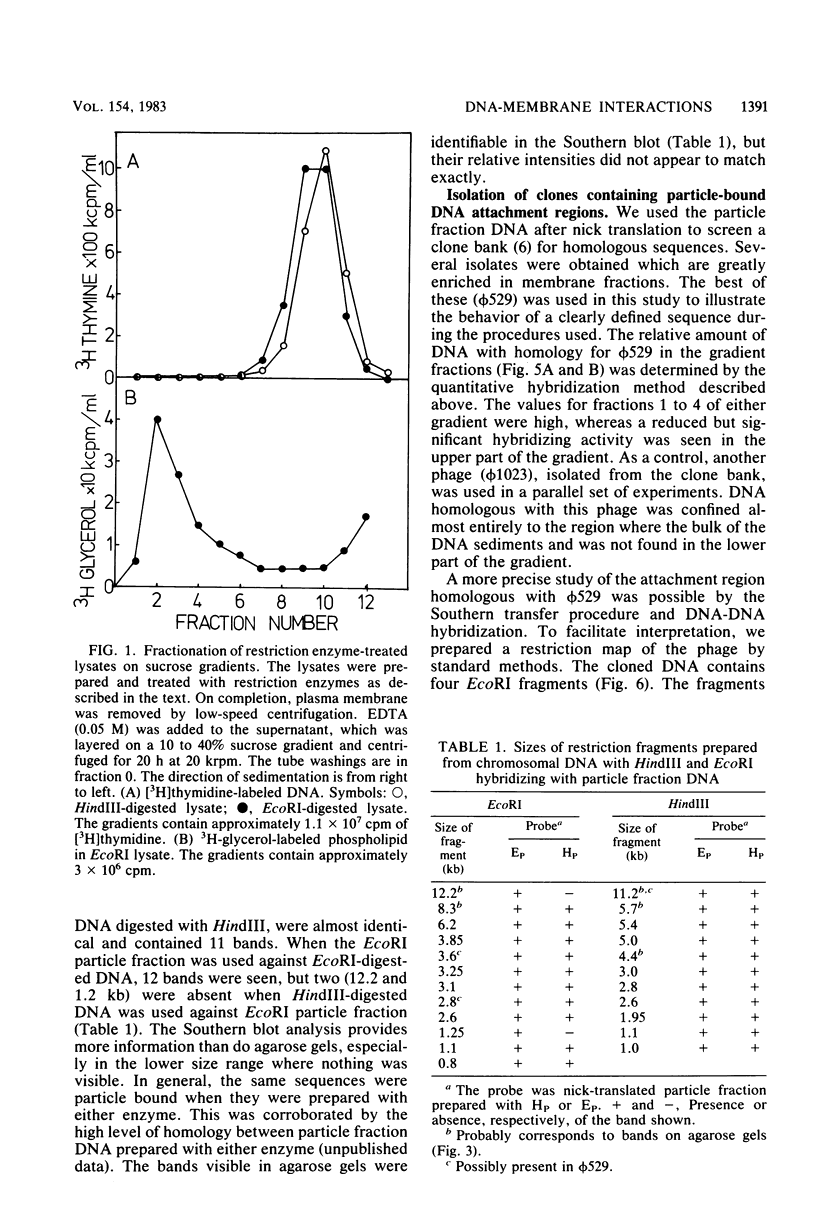
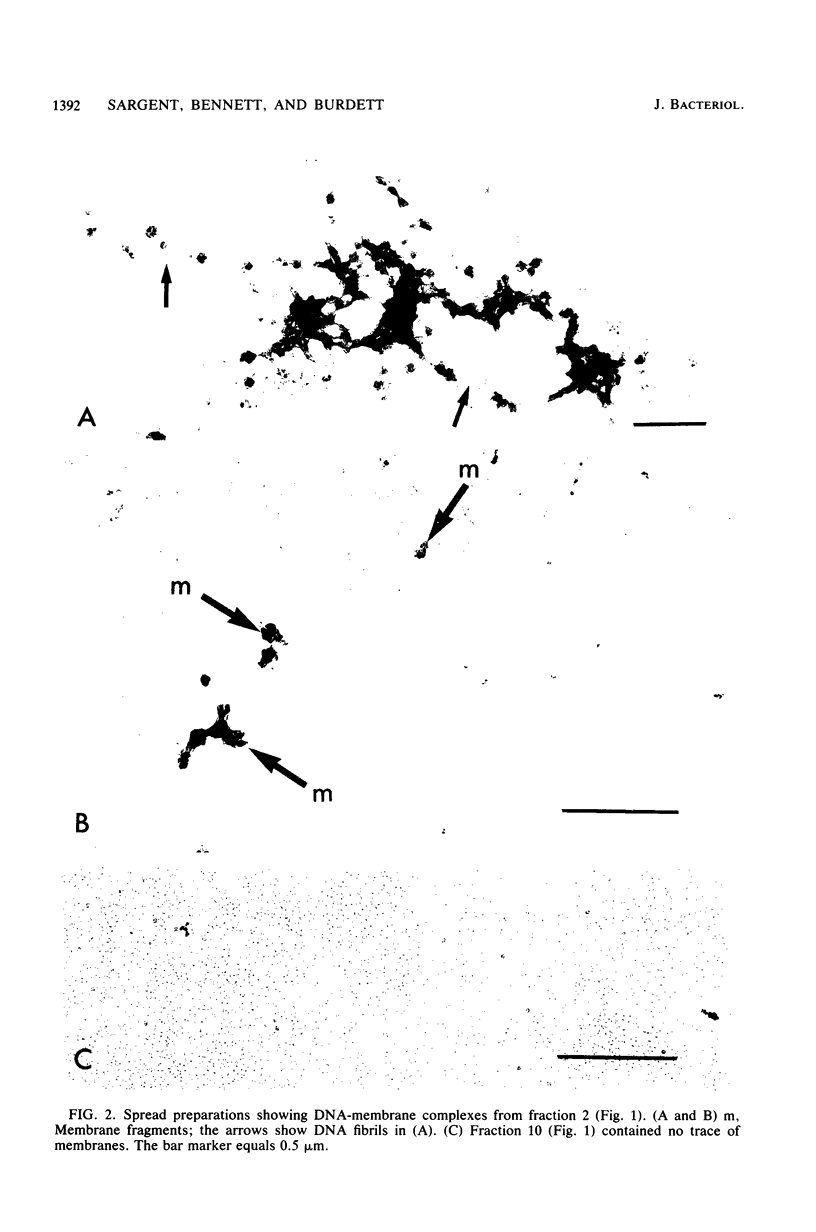
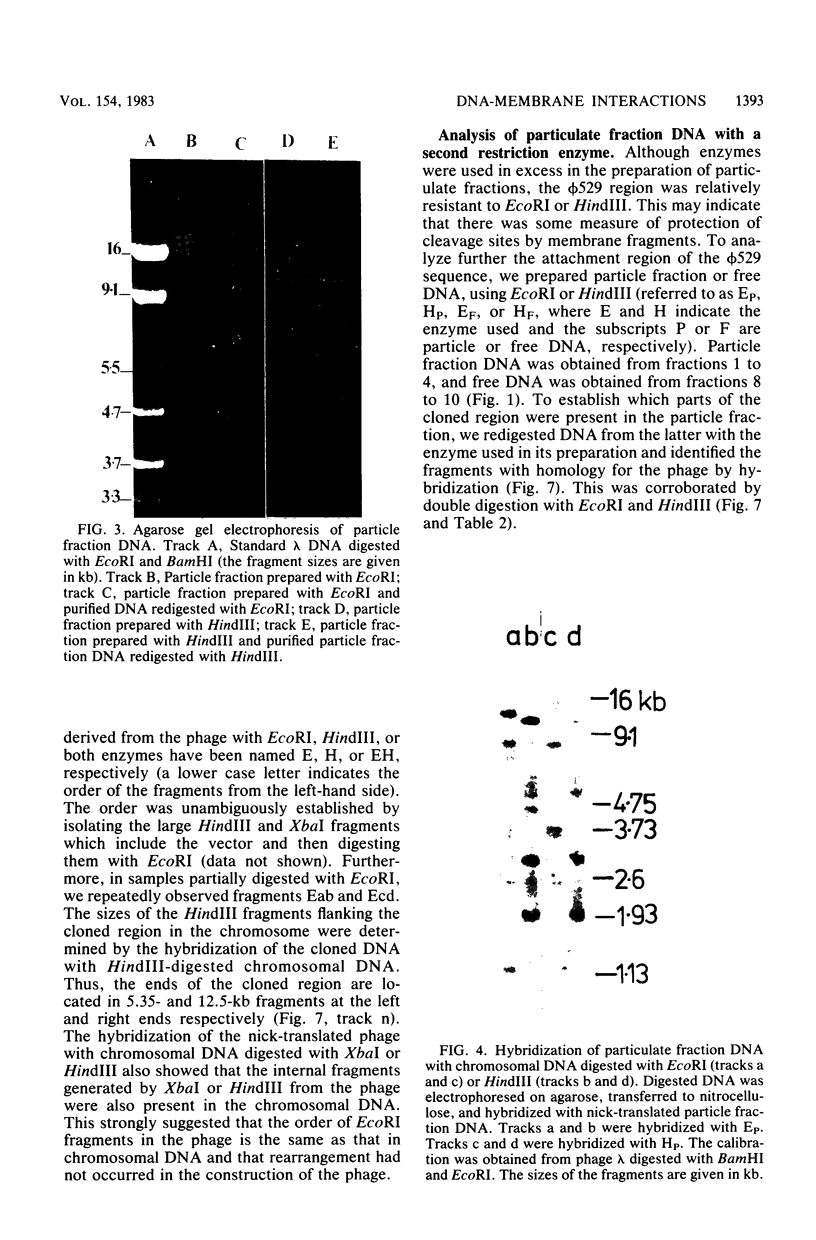
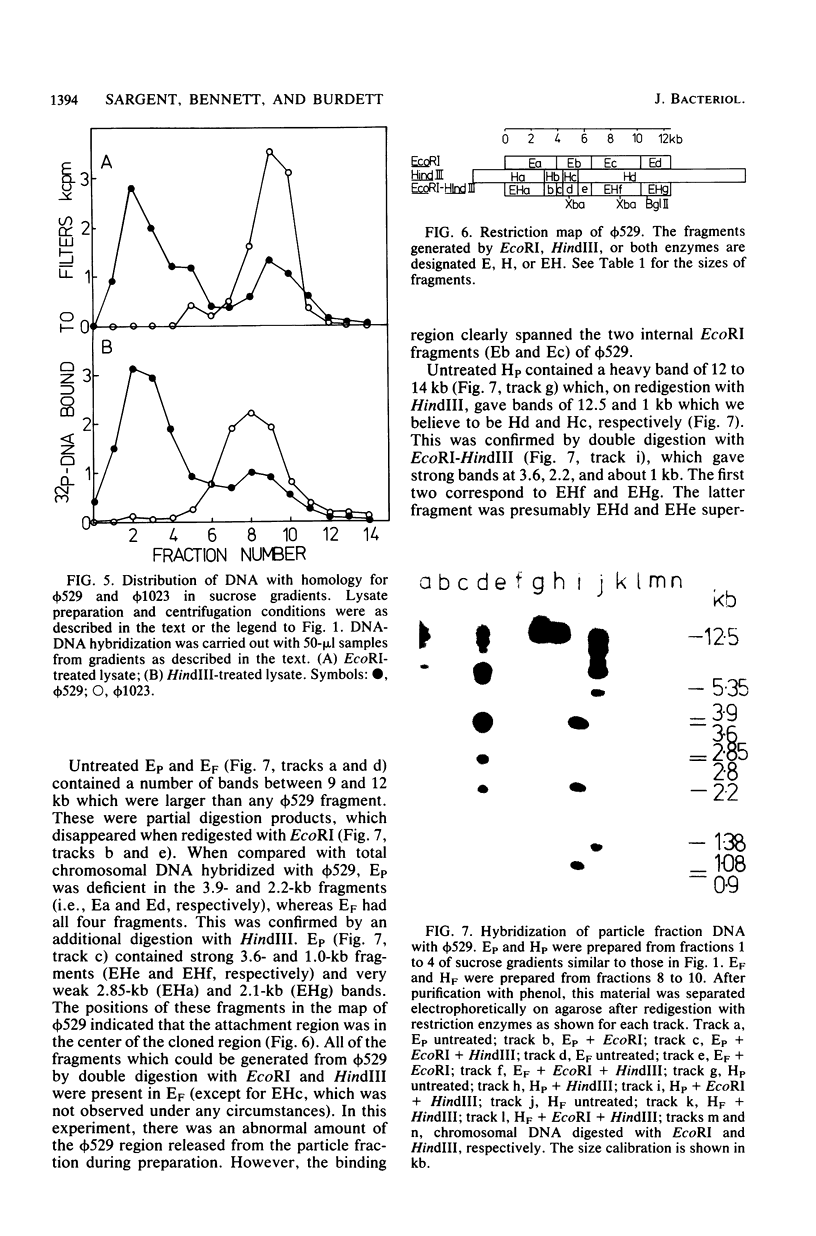
When lysates of Bacillus subtilis were treated with restriction endonucleases EcoRI or HindIII, almost all of the DNA was released from the major plasma membrane fraction that was sedimentable at low speed. However, a very small part of the released DNA, when centrifuged at high speed, appeared to be bound to small membrane fragments. On agarose gels, this material, prepared with either enzyme, contained only a small number of restriction fragments, and the DNA in the sample hybridized with 11 to 12 EcoRI or HindIII fragments of chromosomal DNA. This DNA was used after nick-translation to screen Charon 4A clone banks for phages containing membrane-bound fragments. One of these was studied in detail. Only a part (about 5 kilobases) of the region present in this clone is important in binding the DNA to the membrane subparticle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Brown C., Hendrickson W. G., Boyd D. H., Clifford P., Cote R. H., Schaechter M. Release of Escherichia coli DNA from membrane complexes by single-strand endonucleases. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2756–2760. doi: 10.1073/pnas.74.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Drlica K., Burgi E., Worcel A. Association of the folded chromosome with the cell envelope of Escherichia coli: nature of the membrane-associated DNA. J Bacteriol. 1978 Jun;134(3):1108–1116. doi: 10.1128/jb.134.3.1108-1116.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E., Henner D. J., Hoch J. A. Isolation of Bacillus subtilis genes from a charon 4A library. J Bacteriol. 1981 Apr;146(1):430–432. doi: 10.1128/jb.146.1.430-432.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Pène J. J. Association of many regions of the Bacillus subtilis chromosome with the cell membrane. J Bacteriol. 1973 May;114(2):571–576. doi: 10.1128/jb.114.2.571-576.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Leidner J., Tremblay G. Y. DNA-DNA hybridization on filters at low temperature in the presence of formamide or urea. Biochimie. 1971;53(10):1111–1114. doi: 10.1016/s0300-9084(71)80201-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Hendrickson W., Balakrishnan R., Yamaki H., Boyd D., Schaechter M. Isolation of a replication origin complex from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):262–266. doi: 10.1073/pnas.77.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaidis A. A., Holland I. B. Evidence for the specific association of the chromosomal origin with outer membrane fractions isolated from Escherichia coli. J Bacteriol. 1978 Jul;135(1):178–189. doi: 10.1128/jb.135.1.178-189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. L., Glaser D. A. Effect of growth conditions on DNA-membrane attachment in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2446–2450. doi: 10.1073/pnas.72.6.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Rigney D., Daneo-Moore L., Higgins M. L. Membrane-DNA attachment sites in Streptococcus faecalis cells grown at different rates. J Bacteriol. 1982 Oct;152(1):191–200. doi: 10.1128/jb.152.1.191-200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portalier R., Worcel A. Association of the folded chromosome with the cell envelope of E. coli: characterization of the proteins at the DNA-membrane attachment site. Cell. 1976 Jun;8(2):245–255. doi: 10.1016/0092-8674(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Cavalieri L. F. Shear sensitivity of the E. coli genome: multiple membrane attachment points of the E. coli DNA. Cold Spring Harb Symp Quant Biol. 1968;33:65–72. doi: 10.1101/sqb.1968.033.01.012. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. Attachment of the chromosomal terminus of Bacillus subtilis to a fast-sedimenting particle. J Bacteriol. 1982 May;150(2):623–632. doi: 10.1128/jb.150.2.623-632.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Membrane synthesis in synchronous cultures of Bacillus subtilis 168. J Bacteriol. 1973 Oct;116(1):397–409. doi: 10.1128/jb.116.1.397-409.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. S., Aguinaga M. D., Inman R. B. Isolation of DNA fragments containing replicating growing forks from both E. coli and B. subtilis. Mol Gen Genet. 1981;181(2):241–247. doi: 10.1007/BF00268432. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. S., Inman R. B. Restriction enzyme cleavage of DNA resulting from gently lysed Escherichia coli. Mol Gen Genet. 1978 Nov 9;166(3):245–249. doi: 10.1007/BF00267615. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Masters M. Deoxyribonucleic acid and outer membrane: strains diploid for the oriC region show elevated levels of deoxyribonucleic acid-binding protein and evidence for specific binding of the oriC region to outer membrane. J Bacteriol. 1979 Oct;140(1):50–58. doi: 10.1128/jb.140.1.50-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Yoshikawa H. Chromosome--Membrane association in Bacillus subtilis. III. Isolation and characterization of a DNA-protein complex carrying replication origin markers. J Mol Biol. 1977 Feb 25;110(2):219–253. doi: 10.1016/s0022-2836(77)80070-3. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]