Abstract
Acupuncture has recently been attracting more and more people throughout the world as an alternative treatment, however little is known about its physiological activities (i.e. immune system). We examined acupuncture both quantitatively and qualitatively by measuring CD-positive cell counts and cytokine expression levels in the blood, to determine the activity of T cells, B cells, macrophages and natural killer (NK) cells. Fifteen milliliters of peripheral blood obtained from 17 healthy volunteers aged 21–51 years, were analyzed using flow cytometry before and after acupuncture treatment. There was a statistically significant increase in the number of CD2+, CD4+, CD8+, CD11b+, CD16+, CD19+, CD56+ cells as well as IL-4, IL-1β and IFN-γ levels in the cells after acupuncture stimulation of meridian points. These observations indicate that acupuncture may regulate the immune system and promote the activities of humoral and cellular immunity as well as NK cell activity. In this article, we discussed how acupuncture regulated leukocyte numbers and functions since they are considered to be potential indicators for evaluating complementary and alternative medicine.
Keywords: acupuncture, CD positive cells, cytokine expression, Eastern medicine, leukocyte subset
Introduction
We have previously reported that hot-spring hydrotherapy or walking during a short time could regulate the immune system in humans (1–3). Like hot-spring hydrotherapy, acupuncture treatment is traditionally used to improve disorders or complaints (i.e. pain and so on) of ill subjects (4–7). Therefore, it seems that acupuncture should also have some influence on the immune system as well as the nervous system (8,9). Eastern traditional medicine including acupuncture and moxibustion are once again being recognized in Japan and other Asian countries (10) as well as in the West where it is considered a complementary or alternative medicine. It is considered to be an alternative medicine in the West since it may provide benefits for numerous conditions from common cold to drug addiction and chronic fatigue syndrome (11–13).
The immune system not only works against local infections mediated by pathogens, but also immuno-competent cells and certain cytokines can cause a whole body reaction. Immune system function is closely related to the neuroendocrine immune system (14). A placebo-controlled single-blinded study has shown that acupuncture can cause a neutrophil respiratory burst (15). Thus, we hypothesized that acupuncture may influence the immuno-competent cells qualitatively and quantitatively. Although it is often applied in conjunction with moxibustion, we herein describe how acupuncture alone regulates leukocyte and lymphocyte subpopulations in human peripheral blood.
Methods
Volunteers and Methods of Acupuncture
Seventeen healthy volunteers aged 21–51 years (mean age 35.3 years, 2 men and 15 women) received one acupuncture treatment on 10 August 2005. The exclusion criteria for volunteers were previous episodes of allergic reactions. Disposable acupuncture needles (30 mm, 160 μm in diameter and 50 mm, 230 μm in diameter, Seirin Co. Ltd, Japan) were inserted into the acupuncture points on liver, spleen and kidney meridians as well as into the acupuncture points under the knee joint that could stimulate the internal organs mostly, so called Tai-chi procedure. The points were specified as follows: Ganshu (BL18), Pishu (BL20), Shenshu (BL23) and Zusanli (ST36). Acupuncture treatment was singly performed with needles, which stayed in place for 5 s and were not stimulated, by a well-trained acupuncturist with 30 years of experience. The volunteers did not report any feeling of deqi from the needle.
In a preliminary study, we observed a strong host-response 24 h after a single acupuncture treatment (data not shown). Fifteen milliliters of whole blood were drawn from the forearm veins of all subjects 1 h before acupuncture treatment, 1, 2 and 8 days thereafter, and were collected into standard blood sampling tubes. Every volunteer was informed of the purpose of this trial in advance, and written informed consent was obtained. This research protocol was approved by the Ethics Committee of Kanazawa Medical University.
Leukocyte Counts were Based on the Proportion of Granulocytes and Lymphocytes
Total numbers of leukocytes were recorded with a standard counter. In the differential counting, 200 cells were enumerated on each May–Gruenwald–Giemsa stained slide, and then, each percentage of lymphocytes and granulocytes was determined. The proportion of granulocytes and lymphocytes varies in each subject and susceptibility of the subjects closely related to their proportion (16). High proportion of granulocytes (over 70%) is sympathetic dominant (G type) and that of lymphocytes (over 40%) is parasympathetic dominant (L type). So we performed subgroup analysis based on the proportion of granulocytes and lymphocytes.
Leukocyte Subsets Analyzed by Flow Cytometry
Whole blood was washed 2 times with phosphate buffered saline (PBS). One hundred microliters of the suspensions were stained with 20 μl of fluorescent monoclonal antibodies (anti human CD2+, CD4+, CD8+, CD11b+, CD14+, CD16+, CD19+ and CD56+). Ten thousand stained cells were re-suspended in PBS to detect surface markers by flow cytometry (FACS Calibur; Becton Dickinson Immunocytometry Systems, CA, USA).
Measurement of Cytokine Expression Levels in Blood Cells
Blood cell suspensions were cultured in phorbol 12-myristaten 13-acetate (PMA), ionomycin and bovine serum albumin (BSA) (Sigma Co. Ltd, MO, USA) for 4–5 h at 37°C. Then, cell suspensions were stained with monoclonal antibodies [Percp-CD3, Percp-CD45, FITC-interferon (IFN)-γ, PE-interleukin (IL)-4, FITC-IL-1β]. Ten thousand stained cells were then analyzed by FACS. All antibodies were purchased from Becton Dickinson Immnocytometry System (CA, USA). The typical adjustment of acquisition dot plots for the CD markers and cytokines in FACS analyses was performed in combination with CD11b+/IL-1β, CD4+/IL-4 and CD56+/IFN-γ.
Statistical Analysis
Data are expressed as means ± SD. The association between baseline and changes after acupuncture treatment was analyzed using a one-way analysis of variance (ANOVA) followed by post hoc Dunnett's multiple test. Data analyses were performed on a microcomputer running SPSS version 8.0 (SPSS; IL, USA). A P value <0.05 was considered to be statistically significant.
Results
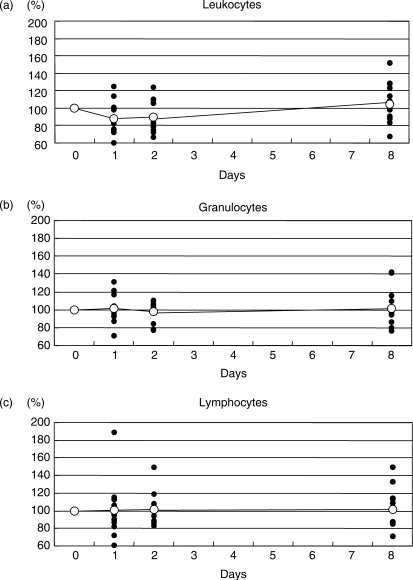
No Significant Changes were Observed in Leukocyte Counts
Numbers of leukocytes were counted 1 h before acupuncture and 1, 2 and 8 days thereafter. We set the cell number observed 1 h before acupuncture at 100% (baseline level) and then, calculated the relative percentage values. The baseline counts of leukocytes were 6191 ± 1274 (mean ± SD, n = 17). No significant changes were observed among the measure of baseline and other measures after acupuncture (Fig. 1a).
Figure 1.
Variations and relative values of leukocytes (a), granulocytes (b) and lymphocytes (c) in all of the volunteers. The solid circles denote the value in each individual and the open circles indicate mean levels on each day. #P < 0.05 versus baseline.
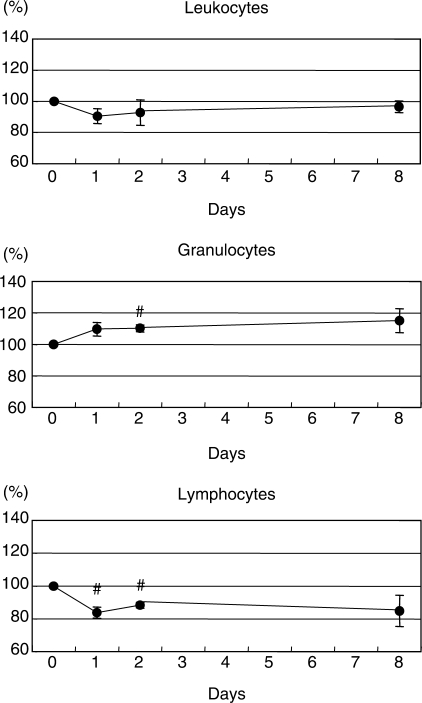
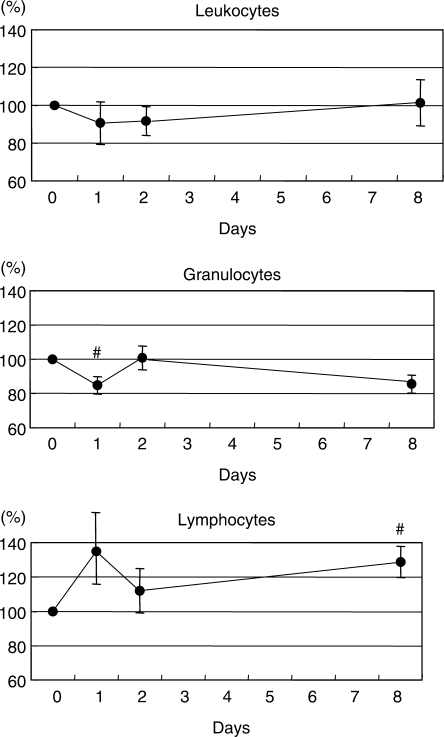
Granulocyte and Lymphocyte Counts Varied According to Group
There was no significant difference in variations of granulocytes and lymphocytes (Fig. 1b and c). In order to clarify this, we performed a subgroup analysis of data based on classifying participants as either granulocyte (G) or lymphocyte (L) types (16). There were five or six subjects classified as either L or G, and six as non-L and non-G. We found that the lymphocyte counts decreased on days 1 and 2, accompanied with an increase of granulocyte numbers on day 2 in the L-type group (P < 0.05, Fig. 2). On the other hand, the granulocyte counts decreased on day 1, whereas the lymphocyte numbers increased on day 8 in the G-type group (P < 0.05, Fig. 3).
Figure 2.
Variations and the relative values of leukocytes in subjects of L-type group. The volunteers were assigned into the G and L-type groups. The solid circles represent means on each day. #P < 0.05 versus baseline.
Figure 3.
Variations and the relative values of leukocytes in subjects of G-type group. The solid circles represent means on each day. #P < 0.05 versus baseline.
Acupuncture Increased the Number of NK Cell-related Subsets
Cell counts of CD2+, CD4+, CD8+, CD11b+, CD16+, CD19+ and CD56+ were evaluated for variations in T cells, B cells, macrophages and NK cells after acupuncture treatment. Our results showed that the CD16+ and CD56+ cell counts, which are closely associated with NK cell activity, increased gradually at statistically significant levels. The variations of CD56+ cell number on day 8 were especially remarkable. The CD2+, CD4+ and CD8+ levels, which are closely associated with activity of T cells, were statistically unchanged except on day 8 after acupuncture (P < 0.05). The CD19+ counts, which are closely associated with activity of B cells, significantly increased on day 8 after acupuncture, like variations of CD16+ and CD56+ counts. The CD11b+ levels, which are closely associated with macrophage activity, showed no significant increase, but the variable patterns were similar to those of CD2+, CD4+ and CD8+ (Table 1).
Table 1.
Variations of CD positive cell counts in each subset of CD2+, CD4+, CD8+, CD11b+, CD16+, CD19+ and CD56+
| Timing for measurement | CD2+ (%) | CD4+ (%) | CD8+ (%) | CD11b+ (%) | CD16+ (%) | CD19+ (%) | CD56+ (%) |
|---|---|---|---|---|---|---|---|
| Before acupuncture | 22.8 ± 2.0 | 8.8 ± 0.8 | 7.0 ± 0.6 | 6.3 ± 1.3 | 0.8 ± 0.2 | 3.7 ± 0.4 | 5.8 ± 0.7 |
| First day after acupuncture | 25.4 ± 2.2 | 9.0 ± 0.9 | 7.5 ± 0.8 | 6.4 ± 1.5 | 1.6 ± 0.3* | 4.0 ± 0.7 | 7.5 ± 1.0* |
| Second day after acupuncture | 19.8 ± 3.7 | 8.9 ± 1.4 | 7.2 ± 1.3 | 7.6 ± 1.6 | 2.2 ± 0.5* | 4.6 ± 1.0 | 8.5 ± 1.5* |
| Eighth day after acupuncture | 34.7 ± 3.5* | 13.6 ± 1.5* | 11.4 ± 1.5* | 7.9 ± 1.5 | 2.1 ± 0.4* | 4.9 ± 0.8* | 11.2 ± 1.8** |
Note. Data are expressed as means ± SD. *P < 0.05, **P < 0.01 compared with baseline levels in each parameter. Blood samples were drawn from a forearm vein with a syringe containing heparin, according to the same procedure as Fig. 1. The blood cell suspensions were stained with fluorescent conjugated monoclonal antibodies and analyzed with fluorescence-activated cell sorter (n = 17).
Cytokine Expression Levels Varied in Blood Cells
To determine whether acupuncture stimulation could affect functional maturation of immuno-competent cells, we investigated expressions of various cytokines in blood cells with FACS analyses. The levels of IL-1β, IL-4 and IFN-γ containing cells, which are closely associated with activities of macrophages and humoral and cellular immunity, were examined.
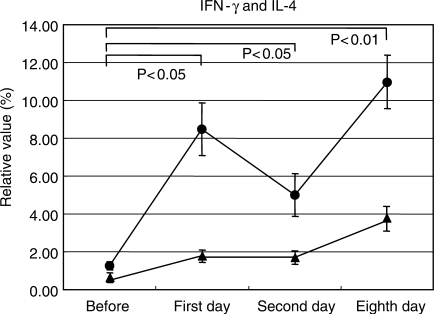
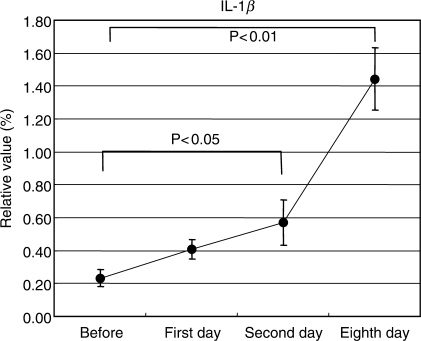
We observed significant increases in the levels of IL-1β, IL-4 and IFN-γ containing cells. However, each variation pattern was different; the levels of IL-4 and IFN-γ increased on days 1 and 2, showing their peak values on day 8 (Fig. 4). In contrast, levels of IL-1β significantly increased on days 2 and 8 after acupuncture (Fig. 5). The expression of IFN-γ increased on days 1 and 2, showing peak values on day 8, and then almost returned to an ordinary level by the 30th day after acupuncture (Fig. 6).
Figure 4.
Variations of interferon (IFN)-γ (circle symbols) and interleukin (IL)-4 (triangle symbols) expression levels in blood cells. To qualitatively evaluate acupuncture, cytokine expression levels in the cells were counted with fluorescence-activated cell sorter. The levels of IFN-γ and IL-4 containing cells were counted as an indicator of cellular (Th1) and humoral (Th2) immunity, respectively. The solid circles represent mean values.
Figure 5.
Variations of interleukin (IL)-1β expression levels in blood cells. To qualitatively evaluate acupuncture, cytokine expression levels in the cells were counted with fluorescence-activated cell sorter. The levels of IL-1β containing cells were counted as an indicator of macrophage. The solid circles represent mean values.
Figure 6.
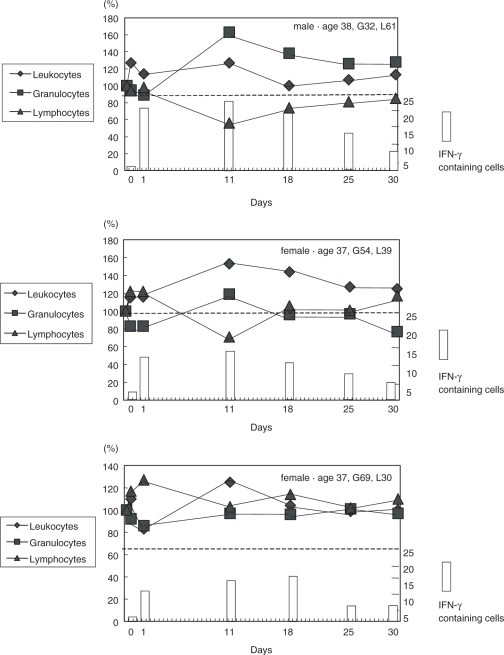
Time course in leukocyte subsets and interferon (IFN)-γ expression levels in blood cells. Diamond symbols denote relative values of leukocytes. Squares symbols show relative values of granulocytes. Triangle symbols indicate relative values of lymphocytes. The columns denote the levels of IFN-γ containing cells.
The Benefits of Acupuncture Lasted for 30 Days after Treatment
To elucidate how long acupuncture stimulation could affect the immune system, three additional healthy volunteers 37–38 years (1 man and 2 women, one L type and two non-L and non-G type), who had never been enrolled in this trial, received acupuncture stimulation, and were followed at intervals different from those of the first 17 volunteers (i.e. on 1 day before acupuncture and on days 1, 11, 18, 25 and 30 thereafter). We found that the expression levels of IFN-γ were most significantly altered on day 11, and that they returned to original levels after 1 month (Fig. 6).
Discussion
We investigated the influence of acupuncture on the immune system, especially on granulocytes, lymphocytes and their subsets. For quantitative evaluation, the number of CD positive cells was counted as indicators of T cells (17), B cells (17), macrophages (18) and NK cells (19). For acceptable qualitative evaluation, antibody titers should be measured as a marker of humoral immunity, and the effectors’ function should be examined as an indicator of cellular immunity. However, we selected the widely used method of examining the cytokine expression levels (20–23). We measured them directly in cytokine containing cells thus eliminating possible artificial factors that could arise from culturing them in test tubes. Because leukocytes have circadian rhythms (24–26), we obtained blood samples at the same time from each donor. The variable pattern of CD11b+ cell counts showing activity of macrophages was similar to those of CD2+, CD4+ and CD8+ cell numbers indicating T-cell activity. The expression levels of IL-1β, a cytokine derived from macrophages, were significantly increased on days 2 and 8 after acupuncture. Ma et al. showed that acupuncture modulated cytokine-NK cell regulatory network (27), and our investigation confirmed the existing IL-1β-macrophage-T cell regulatory network (28–30). Basic research on the cytokine-cell network is an important direction for future research.
We confirmed that acupuncture stimulation could quantitatively as well as qualitatively regulate leukocytes and their subsets. First, the increase of CD2+, CD4+, CD8+, CD11b+, CD16+, CD19+ and CD56+ cell counts as well as the levels of IL-1β, IL-4 and IFN-γ suggested that acupuncture enhances humoral and cellular immunity, including the activities of NK cells (31–35). We observed that the levels of IFN-γ containing cells were increased 9 times in this trial, while in a previous trial on hot-spring bathing, the levels only increased 1.19 times. Based on the observations that expression levels of IFN-γ were elevated on day 11 and returned to the original level by 1 month after stimulation, one treatment of acupuncture per month might be recommended in order to enhance IFN-γ. This remarkable variation was far beyond our prediction. The possible explanation of this enhancement could be activation of the circulatory and/or autonomic nervous systems, even though the details of this mechanism remain unclear. Further research into the mechanism is necessary. Secondly, granulocyte counts were negatively associated with lymphocyte counts. Namely, granulocyte numbers were decreased in subjects with many granulocytes, and were accompanied by an increase in lymphocytes.
The lymphocyte levels, however, decreased in subjects with many lymphocytes, accompanied by an increase in granulocyte counts. Abo and Kumagai (26) reported that granulocytes increased as a result of excitation of the sympathetic nervous system, as were lymphocytes by excitation of parasympathetic nervous system. In this regard, subjects dominated by the sympathetic nervous components could release stress, whereas those in subjects dominated by the parasympathetic nervous system were excited by acupuncture. In this way, cell counts appeared to return to appropriate levels after acupuncture. Another article regarding the role of acupuncture on immune function (31) reported the following considerations: (i) the Zusanli (ST36) point might be one of the specific points modulating immune activity; (ii) this immune modulation system might share a common nervous pathway with the acupuncture analgesia-producing system; (iii) acupuncture treatment might modify NK cell activity through unknown heat-stable humoral factors and the nervous system; (iv) acupuncture might activate the complement system.
In order to prove whether the elevation of leukocyte counts resulted from an infection triggered by acupuncture, the subjects were followed up 8 days after acupuncture. We observed no infectious signs or symptoms including pyodermitis and fever during the follow-up period. Thus, acupuncture treatment might provide maximum benefits without the dangerous side effects such as local infection. We hope that acupuncture treatment can be used more often to treat many disorders including acquired immunodeficiency syndrome.
There are several limitations regarding the protocol of our current investigation. We had no control subjects who did not receive acupuncture treatment. There were also a small number of 17 volunteers who had been enrolled in the study. To demonstrate definitive usefulness of acupuncture stimulation on immune system, double-blind, placebo-controlled studies with a large number of subjects should be required in the future.
Acknowledgments
This study was supported in part by a grant for Japan Society of Acupuncture and Moxibustion. We would also like to thank Patty Christiena Willis for her help in editing this manuscript.
References
- 1.Wang XX, Kitada H, Mastui K, Ohkawa S, Sugiyama T, Kohno Y, et al. Variation of cell populations taking charge of immunity in human peripheral blood following hot spring bathing–quantitative discussion–. J Jpn Assoc Phys Med Balnel Climatol. 1999;62:129–34. [Google Scholar]
- 2.Matsuno H, Wang XX, Wang W-H, Mastui K, Ohkawa S, Sugiyama T, et al. Variation of cell populations taking charge of immunity in human peripheral blood following hot spring bathing–qualitative discussion–. J Jpn Assoc Phys Med Balnel Climatol. 1999;62:135–40. [Google Scholar]
- 3.Wang XX, Katoh S, Liu BX, Ogata, Lai J-E, Matsui K, et al. Effect of physical exercise on leukocyte and lymphocyte subpopulations in human peripheral blood. Cytometry Res. 1998;8:53–61. [Google Scholar]
- 4.Kawakita K. Peripheral mechanisms of acupuncture and moxibustion stimulation and their relations to the characteristics of acupuncture points. J Physiol Soc Jap. 1989;51:303–15. [PubMed] [Google Scholar]
- 5.Shirota A. Fundamental Aspect of Acupuncture. Tokyo: Shunyohdoh; 1940. [Google Scholar]
- 6.Lee JD, Park HJ, Chae Y, Lim S. An overview of bee venom acupuncture in the treatment of arthritis. Evid Based Complement Alternat Med. 2005;2:79–84. doi: 10.1093/ecam/neh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usichenko TI, Hermsen M, Witstruck T, Hofer A, Pavlovic D, Lehmann C, et al. Auricular acupuncture for pain relief after ambulatory knee arthroscopy-a pilot study. Evid Based Complement Alternat Med. 2005;2:185–9. doi: 10.1093/ecam/neh097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma SX. Neurobiology of acupuncture: toward CAM. Evid Based Complement Alternat Med. 2004;1:41–7. doi: 10.1093/ecam/neh017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewith GT, White PJ, Pariente J. Investigating acupuncture using brain imaging techniques: the current state of play. Evid Based Complement Alternat Med. 2005;2:315–9. doi: 10.1093/ecam/neh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YS, Jun H, Chae Y, Park HJ, Kim BH, Chang IM, et al. The practice of Korean medicine: an overview of clinical trials in acupuncture. Evid Based Complement Alternat Med. 2005;2:325–52. doi: 10.1093/ecam/neh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakita K, Shichidou T, Inoue E, Nabeta T, Kitakouji H, Aizawa S, et al. Preventive and curative effects of acupuncture on the common cold: a multicentre randomized controlled trial in Japan. Comp Ther Med. 2004;12:181–8. doi: 10.1016/j.ctim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, Schiff E, Waalen J, Hovell M. Efficacy of acupuncture for treating cocaine addiction: a review paper. J Addict Dis. 2005;24:115–32. doi: 10.1300/j069v24n04_09. [DOI] [PubMed] [Google Scholar]
- 13.Lijue Z. Acupuncture and Chinese patent drugs for treatment of chronic fatigue syndrome. J Tradit Chin Med. 2005;25:99–101. [PubMed] [Google Scholar]
- 14.Landmann RM, Muller FB, Perini C, Wesp M, Erne P, Buhler FR. Changes of immunoregulatory cells induced by psychological and physical stress: relationship to plasma catecholamines. Clin Exp Immunol. 1984;58:127–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Karst M, Scheinichen D, Rueckert T, Wagner T, Wiese B, Piepenbrock S, et al. Effect of acupuncture on the neutrophil respiratory burst: a placebo-controlled single-blinded study. Complement Ther Med. 2003;11:4–10. doi: 10.1016/s0965-2299(02)00117-6. [DOI] [PubMed] [Google Scholar]
- 16.Miyaji C, Watanabe H, Toma H, Akisaka M, Tomiyama K, Sato Y, et al. Functional alteration of granulocytes, NK cells, and natural killer T cells in centenarians. Hum Immunol. 2000;61:908–16. doi: 10.1016/s0198-8859(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 17.Wasik M, Kaczorowska M, Demkow U. Altered expression of immune surface markers in children with recurrent infections of espiratory tract. J Physiol Pharmacol. 2005;56(Suppl 4):237–43. [PubMed] [Google Scholar]
- 18.He W, Qiang M, Ma W, Valente AJ, Quinones MP, Wang W, et al. Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther. 2006;17:949–59. doi: 10.1089/hum.2006.17.949. [DOI] [PubMed] [Google Scholar]
- 19.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–6. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 21.Garside P, Mowat AM. Polarization of Th-cell responses: a phylogenetic conseqence of nonspecific immune defense? Immunol Today. 1995;16:220–3. doi: 10.1016/0167-5699(95)80162-6. [DOI] [PubMed] [Google Scholar]
- 22.Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–9. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 23.Wegmann T, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 24.Toyabe S, Iiai T, Fukuda M, Kawamura T, Suzuki S, Uchiyama M, et al. Identification of nicotinic acetylcholine receptors on lymphocytes in the periphery as well as thymus in mice. Immunology. 1997;92:201–5. doi: 10.1046/j.1365-2567.1997.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abo T, Kawate T, Itoh K, Kumagai K. Studies on the bioperiodicity of the immune response. I. Circadian rhythms of human T, B and K cell traffic in the peripheral blood. J Immunol. 1981;126:1360–3. [PubMed] [Google Scholar]
- 26.Abo T, Kumagai K. Studies of surface immunoglobulins on human B lymphocytes. III. Physiological variations of SIg+ cells in peripheral blood. Clin Exp Immunol. 1978;33:441–52. [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Z, Wang Y, Fan Q. The influence of acupuncture on interleukin 2 interferon-natural killer cell regulatory network of kidney-deficiency mice. Zhen Ci Yan Jiu. 1992;17:139–42. [PubMed] [Google Scholar]
- 28.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: the ultimate antagonist (I) Evid Based Complement Alternat Med. 2006;3:25–30. doi: 10.1093/ecam/nek022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: modulating allergic and infectious disease pathology (II) Evid Based Complement Alternat Med. 2006;3:209–15. doi: 10.1093/ecam/nel020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: modulating tumor immunity, autoimmunity and alloreactive immunity (III) Evid Based Complement Alternat Med. 2006;3:309–16. doi: 10.1093/ecam/nel047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaout MA. Structure and function of the leukocyte adhesion molecule CD11/CD18. Blood. 1991;75:1037–50. [PubMed] [Google Scholar]
- 33.Evans DL, Folds JD, Petitto JM, Golden RN, Pedersen CA, Corrigan M, et al. Circulating natural killer cell phenotypes in men and women with major depression. Arch Gen Psychiatry. 1992;49:388–95. doi: 10.1001/archpsyc.1992.01820050052009. [DOI] [PubMed] [Google Scholar]
- 34.Evans DL, Pedersen CA, Folds JD. Major depression and immunity—preliminary evidence of decreased natural killer cell populations. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:739–48. doi: 10.1016/0278-5846(88)90019-x. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Yu Y, Guo SY, Kasahara T, Hisamitsu T. Acupuncture stimulation enhances splenic natural killer cell cytotoxicity in rats. Jpn J Physiol. 1996;46:131–6. doi: 10.2170/jjphysiol.46.131. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Kasahara T, Sato T, Asano K, Yu G, Fang J, et al. Role of endogenous interferon-gamma on the enhancement of splenic NK cell activity by electroacupuncture stimulation in mice. J Neuroimmunol. 1998;90:176–86. doi: 10.1016/s0165-5728(98)00143-x. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Toyabe S, Moroda T, Tada T, Tsukahara A, Iiai T, et al. Circadian rhythm of leucocytes and lymphocytes subsets and its possible correlation with the function of the autonomic nervous system. Clin Exp Immunol. 1997;110:500–8. doi: 10.1046/j.1365-2249.1997.4411460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]