Abstract
We show that ants can reconnoitre their surroundings and in effect plan for the future. Temnothorax albipennis colonies use a sophisticated strategy to select a new nest when the need arises. Initially, we presented colonies with a new nest of lower quality than their current one that they could explore for one week without a need to emigrate. We then introduced a second identical low quality new nest and destroyed their old nest so that they had to emigrate. Colonies showed a highly significant preference for the (low quality) novel new nest over the identical but familiar one. In otherwise identical experiments, colonies showed no such discrimination when the choice was between a familiar and an unfamiliar high-quality nest. When, however, either all possible pheromone marks were removed, or landmarks were re-orientated, just before the emigration, the ants chose between identical low-quality new nests at random. These results demonstrate for the first time that ants are capable of assessing and retaining information about the quality of potential new nest sites, probably by using both pheromones and landmark cues, even though this information may only be of strategic value to the colony in the future. They seem capable, therefore, of latent learning and, more explicitly, learning what not to do.
Keywords: collective decision-making, house-hunting, memory, planning for the future, public and private information, Temnothorax albipennis
1. Introduction
A reconnaissance is an examination of a region to ascertain strategic features through a preliminary survey (Oxford English Dictionary). Thus, reconnaissance is usually associated with planning and foresight. As such, reconnaissance would seem to be an advanced form of behaviour and certainly beyond the scope of a mere reflex automaton. Here, we test experimentally the ability of ants to reconnoitre.
Rats (Rattus rattus) and honeybees (Apis mellifera) reconnoitre. Rats can learn unrewarded mazes (Blodgett 1929). Honeybees, on first leaving the hive, learn landmarks on exploratory flights and return home before beginning their first foraging bouts (Menzel et al. 2000; Menzel 2001). Both of these examples are considered to be clear cases of latent learning, i.e. gathering and storing information, without an immediate reward, in case it is useful later (Thorpe 1963). Latent means hidden. In these cases of so-called latent learning, what is hidden is not so much the learning as the form of the possible or potential reward.
We used the classical model of house-hunting in Temnothorax albipennis ants to determine if ants are able to reconnoitre and exhibit latent learning. In nature these ants live in flat rock crevices. When these nests are damaged beyond repair the ants have to find a new nest to which the whole colony can emigrate. T. albipennis colonies can be housed in the laboratory in nests formed from two microscope slides held apart by a cardboard perimeter. When such a nest is made uninhabitable by removing the top microscope slide, the ants are able to select the best available new nest site (Franks et al. 2003b). They evaluate a comprehensive set of criteria when selecting a new nest. For example, they measure floor area (Mallon & Franks 2000), headroom and entrance size (Franks et al. 2003b, 2006b), the darkness of the nest cavity (Franks et al. 2003b, 2006b), the state of hygiene of the new nest cavity (Franks et al. 2005) and even the proximity of hostile conspecific neighbours (Franks et al. 2007). Indeed, experiments strongly suggest that these ants use a weighted additive strategy, the most comprehensive of consumer evaluations, to choose nests with the best combination of attributes (Franks et al. 2003b). Individual workers initially evaluate nests alone, hesitating longer to recruit to less desirable ones but switching to recruitment by tandem running more quickly if they encounter better nest sites (Mallon et al. 2001). An ant led to a new nest site by a tandem run probably makes its own evaluation and may recruit others in turn by leading new tandem runs (Pratt et al. 2002). When the ants discover a sufficient number of their nest mates in a new nest site, i.e. a quorum, they engage the colony in a full emigration by carrying their nest mates (Mallon et al. 2001). Such carrying is about three times faster than tandem running (Pratt et al. 2002).
All of the above suggest that nest evaluation is a relatively slow and an extremely thorough activity. The ants can decide more quickly under harsh conditions, but such speed is at the cost of accuracy (Franks et al. 2003a). These ants will also move to improve, i.e. they will emigrate to a much better nest if one is available even if their old nest is intact (Dornhaus et al. 2004). In such circumstance, the ants take their time and use a very high quorum threshold (Dornhaus et al. 2004). This implies that some individuals survey new nest sites without the immediate need for an emigration.
Here we pose the question: do ants make a preliminary survey of the housing stock in their neighbourhood in case they need to emigrate later? If they do, we would predict that they would react differently to a familiar nest site than to an identical one that had just been introduced when their old nest was destroyed. Such behaviour might save time during the emergency of homelessness and would be a clear example of reconnaissance. If the ants do react differently, by choosing or rejecting a familiar new nest over an identical novel one, they may have learned about the familiar nest and retained this information for potential future benefit. We also investigated the possible cues the ants might use to remember nests.
2. Material and methods
We performed experiments in which colonies could examine a new nest site (nest 1) for one week. In experiments 1, 3, 4 and 5, nest 1 was of lower quality than the nest they currently inhabited. At the end of the week, an identical and equidistant new nest (nest 2) was introduced. Immediately after this second new nest was introduced, the colony's old nest was destroyed and the colony had to emigrate. Since nests 1 and 2 are of identical quality, we would expect the colonies to choose randomly between them if they had not learned about nest 1. If the colonies show a significant preference for either the first or the second new nest, this would be evidence that they had indeed reconnoitred the first new nest and retained some information about it.
We presented colonies with choices between potential new nest sites following the well-established protocol described in detail in Franks et al. (2003a,b, 2006b). The experiments were performed in large Petri dishes (figure 1). Nests were made of a cardboard perimeter sandwiched between two glass slides. In all of the experiments, the old nest was destroyed by removing the top glass slide and food trays, and water tubes were removed during the emigrations. The positions of the two new nests were randomized, i.e. which was to the left and which to the right, in each experiment. After 24 h, we recorded which new nest the colony had chosen. Colonies were only deemed to have ‘chosen’ a nest if all of the brood was in that nest. If even a single brood item was in the alternative nest the colony was deemed to have split.
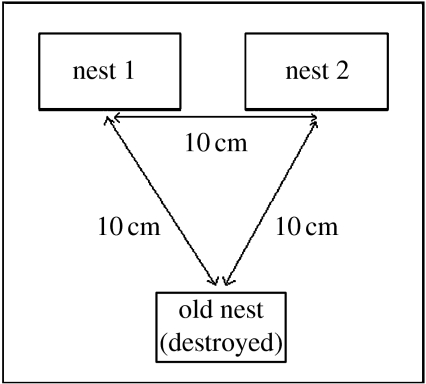
Figure 1.
Diagram of the arena setup used in all experiments. Nest 1 is introduced first and nest 2 is introduced a week later. The location of nest 1 (to the left or right) was randomized. The entrances of the nests are 10 cm apart.
We employed nest sites of three different qualities in our experiments: ‘mediocre’, ‘good’ and ‘superior’. The ants' preference ranking of such nests is well established (Franks et al. 2003b). All nests had an internal area of 33×55 mm and an entrance of 2 mm width. Both the good and mediocre nests had 1.1 mm headroom, with the good nest having an additional cover so that the region inside was dark. Superior nests were also covered and had 1.8 mm of headroom. In all experiments, except number 2, ants were housed in good nests while both newly introduced nests were of mediocre quality (table 1). This ensured that the ants would not move to improve during week 1 (Dornhaus et al. 2004).
Table 1.
The colonies' nest choices 24 h after the destruction of the old nest. ‘Experimental setup’ summarizes the design of the experiments (given in more detail in the text): Both new nests were either of mediocre quality and inferior to the old nest (MQ) or they were of the same superior quality (SQ) as the old nest; colonies were disturbed (D); pheromones were removed (P); landmarks were reoriented (L). Experiments 1 and 2 were run with a different set of colonies than experiments 3–5. p-values were obtained from cumulative binomial tests of the number of colonies that chose nest 1 versus the number that chose nest 2. For the purposes of the binomial test, it was assumed that the probability of a colony choosing nest 1 or nest 2 was 0.5.
| nest choice | ||||||
|---|---|---|---|---|---|---|
| experiment | experimental setup | nest 1 | nest 2 | split | N | p-value |
| 1a | MQ | 2 | 23 | 5 | 30 | <0.0001 |
| 1b | MQ, D, L | 10 | 7 | 9 | 26 | 0.6291 |
| 1c | MQ | 3 | 17 | 3 | 24a | 0.0026 |
| 2 | SQ | 1 | 0 | 23 | 30b | 1.0000 |
| 3 | MQ, D, L | 16 | 17 | 3 | 36 | 1.0000 |
| 4 | MQ, D, P | 20 | 14 | 2 | 36 | 0.3915 |
| 5 | MQ, D | 11 | 25 | 0 | 36 | 0.0288 |
One colony which had not moved after 24 h was excluded from the analysis.
Six colonies which emigrated into nest 1 prior to the introduction of nest 2 were excluded from the analysis.
We ran experiment 1 thrice with slight alterations (table 1). In experiment 1a, we did not photograph the nests during the exploratory phase, but simply recorded the final choice of nest of each colony. In experiment 1b, we photographed the new nest site during the first hour of week 1 and both new nest sites during the first hour of week 2. To make these photographs, we moved each large Petri dish arena to the camera setup, thereby moving the landmarks the ants might have been using for orientation (Pratt et al. 2001; McLeman et al. 2002). Photographs were taken at 5 min intervals so that we could count the number of ants exploring the new potential nest site or sites. In experiment 1c, we also took photographs, but we did not move the Petri dishes. Instead, we photographed the Petri dishes in situ so that familiar landmarks remained.
In experiment 2, we used the same basic procedure but without photographing the nests or moving the Petri dishes. However, all of the nests, the old and both new ones, were of superior quality. We housed the ants in a superior quality nest to minimize the possibility that the ants would move to improve during week 1.
After our first results indicated that the ants discriminate against mediocre familiar nests, we ran additional experiments with new colonies, to begin to elucidate the mechanisms used by the ants. In all of the following experiments (3, 4, & 5), the floor of each Petri dish arena was carpeted wall-to-wall with an acetate sheet. In experiment 3, we repeated the basic design of experiments 1a and 1c, but we changed the location of landmarks in and around the experimental Petri dishes by rotating all of the contents of the Petri dish, i.e. the acetate floor and the old and familiar nests, through 180° just before the old nest was destroyed and the second available nest was introduced. We used this design (instead of simply rotating the Petri dish and its contents) in case the ants might use any irregularities on the inside walls of the Petri dishes as landmarks. We were careful to position the old and familiar nests exactly atop their original positions on the rotated acetate sheet, so that any pheromone trails would remain aligned. In experiment 4, we also repeated the basic design of experiments 1a and 1c, but removed any possible pheromone marking of the arena and the familiar new nest by removing the acetate floor sheet and replacing both it and the familiar new nest with otherwise identical new ones. In this way, at the time the old nest was destroyed both of the available nests were entirely new and neither they nor the arena floor, between them and the old nest, had any pheromone marks. In a final trial, experiment 5, we repeated the original experiments 1a & 1c to determine if these new colonies also discriminated against familiar mediocre nests. However, in experiment 5 we also used an acetate sheet on the floor of each Petri dish during the familiarization week. Just before the emigrations, this sheet and all the nests were lifted and replaced exactly where they had been. Hence, experiment 5 serves as a control for any disturbance in experiments 3 and 4.
3. Results
Table 1 presents the results of experiments 1 to 5. In experiment 1a, 1c & 5, when familiar cues such as landmarks and pheromone marks remained, the ant colonies rejected the mediocre nest 1, the new potential nest site that they had had a week to explore, in favour of the structurally identical, and therefore also mediocre alternative, nest 2, that had been introduced only when their old nest had been destroyed. In experiment 1b & 3, where the landmarks around the arena had been changed, the ants showed no preference for either nest 1 or nest 2. Similarly, when all possible pheromone marks on the arena floor or in nest 1, from the initial familiarization week, were removed (experiment 4) the ants also did not significantly prefer or discriminate against either of the new nests. Experiment 5 suggests that minor disturbance does not cause the ants to choose at random.
In experiment 2, the ants showed no preference for either of the alternative new superior quality nests, even though the positions of landmarks and pheromone trails were held constant in this experiment.
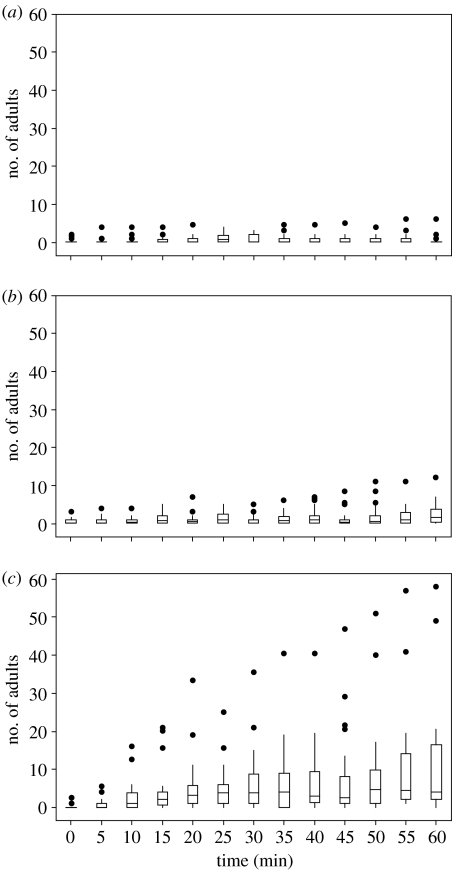
Figure 2 shows the number of workers exploring the potential new nest sites in experiment 1c. It is clear that a small number of workers explore the new nest site in the first hour of week 1—but these numbers do not grow over the hour of observation. In the first hour of week 2, both new nests are explored more, probably because the ants are now homeless. In both of the new nests, the number of exploring workers increase over the hour but they do so more rapidly in nest 2. Indeed, the numbers in the newly available nest grow rapidly up to and beyond the typical quorum threshold of between 5 and 17 ants (Pratt et al. 2002; Dornhaus et al. 2004; Franks et al. 2006a). Proximately, this accounts for the ants choosing these new novel nests in preference to the structurally identical familiar ones.
Figure 2.
Nest visitation data from experiment 1c, showing the numbers of adults in each nest at each 5 min interval in the first hour of the weeks 1 and 2. The horizontal line within the interquartile range of each rectangle represents the median, vertical whiskers extend to ±1.5×interquartile range and outliers are represented by solid circles. (a) Nest 1 at the beginning of week 1. Linear regression of the medians versus time shows that the relationship is best described by the equation: median=0.082−0.00082×time (r2=0.6%, n=13, p=0.802 and s.e. of the slope=0.0032), i.e. there is no relationship between the number of adults and time. (b) Nest 1 at the beginning of week 2, immediately after the introduction of the novel nest 2. Linear regression describes the relationship as median=0.184+0.0157×time (r2=45.7%, n=13, p=0.011 and s.e. of the slope=0.0051), i.e. there is a relationship between the number of adults and time. (c) Nest 2 at the beginning of week 2, immediately after the introduction of the novel nest 2. Linear regression describes the relationship as median=0.739+0.0690×time (r2=69.2%, n=13, p<0.001, s.e. of the slope=0.0139), i.e. there is a relationship between the number of adults and time. For the 60 min following the destruction of their old nest, many more ants explore (and occupy) nest 2 than nest 1, even though both of these nests are of identical low quality.
4. Discussion
The results of our experiments indicate that these ants engage in reconnaissance and retain the information thus gathered. In experiments 1a & 1c, when landmarks and pheromone trails were unaltered and there was little disturbance, the ants very clearly discriminated between the new nests 1 and 2 in favour of nest 2. The minor disturbance involved in the replicate experiment 5 did not change the significance of the result (table 1). Nests 1 and 2 were identical except that the ants could become familiar with nest 1 for one week when they had no need to emigrate. The ants have gathered and retained information about this nest without an obvious immediate need or reward. Thus, they seem able to retain information of little current value for later use.
The fascinating aspect of this behaviour is that when a familiar potential new nest is of lower quality than their current nest, the ants use their earlier reconnaissance to reject this nest. In this way, they can focus their efforts on searching for a better (or indeed here any newer) alternative. Our findings suggest that the ants discriminate against a familiar poor-quality nest using a combination of learned landmarks and pheromone markings. When either landmarks were changed or pheromone marks removed, the ants showed no nest preferences (table 1). The tendency towards choosing the familiar nest 1 when pheromones were removed, but landmarks remained unaltered (experiment 4, table 1), suggests that the ants might be rejecting the familiar nest on the basis of negative pheromone marking (Robinson et al. 2005; see also Fourcassié & Deneubourg 1992). This is further supported by the result (experiment 2) that the ants show no preferences if both new nests are of high quality and/or similar to the nest the ants were inhabiting, as such nest sites should not be marked adversely. However, the ants also choose randomly between the two new nests when the pheromones are retained but the landmarks are altered (experiment 3, table 1). This indicates that the learning of landmarks also plays a role in the ants retaining information about a known nest site. While negative pheromone markings should be available to all colony members, only scouts who have visited nest 1 during the initial week should know the associated landmarks. We think, therefore, that the ants not only reconnoitre and possibly make the information of a poor new nest site available to their nest-mates via pheromone markings, but also that some individuals latently learn the landmarks leading to a potential new nest site. In this sense, the ants might be using information that is both public (pheromones) and private (landmarks). Although extreme hygiene problems, in the form of dead nest mates, can cause the ants to reject a nest (Franks et al. 2005), we doubt that the mere exploration of a nest would contaminate it. This is supported by the results of experiment 2.
We found these results surprising. One might have expected the ants always to use their reconnaissance and latent learning to provide them with information on any suitable nest site that they could use during the emergency of homelessness. For this reason, we expected the ant colonies to choose the familiar nest 1 in preference to nest 2. Instead, they rejected the familiar inferior alternative (nest 1) in favour of the not previously evaluated nest 2. This is a beautiful example of the ants not taking the first option but being prepared to explore for better alternatives. In this sense, we do not regard the ants' choice of a novel, but otherwise identical nest, as irrational. It might be beneficial to ignore a familiar and not very attractive alternative to search elsewhere and accept an identical, but more novel, alternative. More ants may have more up to date information on the novel alternative and it is not a worse option. Alternatively, marking and remembering a poor nest may curtail further wasteful surveying of it when the ants have no need to emigrate. Then during homelessness, many of the ants that visit the familiar nest 1 might do so only briefly because they find that they already know about it, and as a result the quorum threshold is not achieved in nest 1, resulting in the choice of nest 2 via a form of ‘sensory trap’ (Tinbergen 1953).
One interesting aspect of latent learning is the predictability of the potential reward in space and time. A honeybee should clearly learn landmarks during an exploratory flight (Menzel et al. 2000; Menzel 2001) in order to be able to return to the safety of the hive and then engage in successful foraging. Here, the potential benefit seems to be very predictable, with the collected information likely to be used very soon (on the next foraging flight). By contrast, rats might learn so-called unrewarded mazes (Blodgett 1929) for escape routes, just in case they encounter one of their natural enemies, or to find food or a new nest if and when such rewards are available. Here, the rat might be investing in latent learning with no pay-off at all (if the situations above never arise) or only one at some indeterminate time in the future. For these ants, in nature, the benefit of ignoring a familiar poor nest to search elsewhere will depend on the relative abundance of better ones.
Recently, Raby et al. (2007) have shown that western scrub-jays (Aphelocoma californica) plan for the future by storing particular foods where they will be needed most. This might even involve ‘mental time travel’ because it seems to involve (i) novel behaviour based on learning and (ii) the animal anticipating a different motivational state to its current one. However, our findings suggest that ants may also in effect anticipate the future through individuals acquiring private information by learning landmarks and providing public information in the form of repulsive pheromones. Moreover, the motivational states of the different sets of ants depositing pheromones, when the colony does not need to emigrate, and those reacting to them, when the colony does need a new home, are likely to be different. So planning for the future can be a social activity based on relatively simple rules without any form of mental time travel. Put simply, latent learning associated with exploration can have dividends in the future.
Here, we have shown reconnaissance and latent learning in an ant species. The ants use the information they acquire potentially to perform better by discriminating against certain alternatives and thus knowing what not to do.
Acknowledgments
We thank Mike Mogie, Tim Kovacs, Alan Roberts and all the members of the University of Bristol Ant Lab for helpful discussion and comments. N.R.F. wishes to thank both the BBSRC (E19832) and the EPSRC (GR/S78674/01) for supporting his research. A.D. thanks the German Science Foundation (DFG, Emmy Noether fellowship). S.M.B. thanks the Leverhulme trust for supporting her research in Bristol.
References
- Blodgett H.C. The effect of the introduction of reward upon the maze performance of rats. Univ. Calif. Publ. Psychol. 1929;4:113–134. [Google Scholar]
- Dornhaus A, Franks N.R, Hawkins R.M, Shere H.N.S. Ants move to improve—colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim. Behav. 2004;67:959–963. doi:10.1016/j.anbehav.2003.09.004. [Google Scholar]
- Fourcassié V, Deneubourg J.L. Collective exploration in the ant Monomorium pharaonis L. In: Billen J, editor. Biology and evolution of social insects. Leuven University Press; Leuven, Belgium: 1992. pp. 369–373. [Google Scholar]
- Franks N.R, Dornhaus A, Fitzsimmons J.P, Stevens M. Speed versus accuracy in collective decision-making. Proc. R. Soc. B. 2003a;270:2457–2463. doi: 10.1098/rspb.2003.2527. doi:10.1098/rspb.2003.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R, Mallon E.B, Bray H.E, Hamilton M.J, Mischler T.C. Strategies for choosing among alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 2003b;65:215–223. doi:10.1006/anbe.2002.2032. [Google Scholar]
- Franks N.R, Hooper J.W, Webb C, Dornhaus A. Tomb evaders: house-hunting hygiene in ants. Biol. Lett. 2005;1:190–192. doi: 10.1098/rsbl.2005.0302. doi:10.1098/rspb.2005.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R, Dornhaus A, Best C.S, Jones E.L. Decision-making by small & large house-hunting ant colonies: one size fits all. Anim. Behav. 2006a;72:611–616. doi:10.1016/j.anbehav.2005.11.019 [Google Scholar]
- Franks N.R, Dornhaus A, Metherell B.G, Nelson T.R, Lanfear S.A.J, Symes W.S. Not everything that counts can be counted: Ants use multiple metrics for a single nest trait. Proc. R. Soc. B. 2006b;273:165–169. doi: 10.1098/rspb.2005.3312. doi:10.1098/rspb.2005.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R, Dornhaus A, Hitchcock G, Guillem R, Hooper J, Webb C. Avoidance of conspecific colonies during nest choice by ants. Anim. Behav. 2007;73:525–534. doi:10.1016/j.anbehav.2006.05.020 [Google Scholar]
- Mallon E, Franks N.R. Ants estimate area using Buffon's needle. Proc. R. Soc. B. 2000;267:765–770. doi: 10.1098/rspb.2000.1069. doi:10.1098/rspb.2000.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon E.B, Pratt S.C, Franks N.R. Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2001;50:352–359. doi:10.1007/s002650100377. [Google Scholar]
- Menzel R, Brandt R, Gumbert A, Komischke B, Kunze J. Two spatial memories for honeybee navigation. Proc. R. Soc. B. 2000;267:961–968. doi: 10.1098/rspb.2000.1097. doi: 10.1098/rspb.2000.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem. 2001;8:53–62. doi: 10.1101/lm.38801. doi:10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- McLeman M.A, Pratt S.C, Franks N.R. Navigation using visual landmarks by the ant Leptothorax albipennis. Insectes Sociaux. 2002;49:203–208. doi:10.1007/s00040-002-8302-2. [Google Scholar]
- Pratt S.C, Brooks S.E, Franks N.R. The use of edges in visual navigation by the ant Leptothorax albipennis. Ethology. 2001;107:1125–1136. doi: 10.1046/j.1439-0310.2001.00749.x [Google Scholar]
- Pratt S.C, Mallon E.B, Sumpter D.J.T, Franks N.R. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2002;52:117–127. doi:10.1007/s00265-002-0487-x. [Google Scholar]
- Raby C.R, Alexis D.M, Dickson A, Clayton N.S. Planning for the future by western scrub-jays. Nature. 2007;445:919–921. doi: 10.1038/nature05575. doi:10.1038/nature005575 [DOI] [PubMed] [Google Scholar]
- Robinson E.J.H, Jackson D.E, Holcombe M, Ratnieks F.L.W. Insect communication—no entry signal in ant foraging. Nature. 2005;438:442. doi: 10.1038/438442a. doi:10.1038/438442a. [DOI] [PubMed] [Google Scholar]
- Thorpe W.H. 1st edn. Methuen; London, UK: 1963. Learning and instinct in animals. [Google Scholar]
- Tinbergen N. Collins; London, UK: 1953. The Herring gull's world. [Google Scholar]