Abstract
In social insects, colonies may contain multiple reproductively active queens. This leads to potential conflicts over the apportionment of brood maternity, especially with respect to the production of reproductive offspring. We investigated reproductive partitioning in offspring females (gynes) and workers in the ant Formica fusca, and combined this information with data on the genetic returns gained by workers. Our results provide the first evidence that differential reproductive partitioning among breeders can enhance the inclusive fitness returns for sterile individuals that tend non-descendant offspring. Two aspects of reproductive partitioning contribute to this outcome. First, significantly fewer mother queens contribute to gyne (new reproductive females) than to worker brood, such that relatedness increases from worker to gyne brood. Second, and more importantly, adult workers were significantly more related to the reproductive brood raised by the colony, than to the contemporary worker brood. Thus, the observed breeder shift leads to genetic benefits for the adult workers that tend the brood. Our results also have repercussions for genetic population analyses. Given the observed pattern of reproductive partitioning, estimates of effective population size based on worker and gyne samples are not interchangeable.
Keywords: kin selection, reproductive skew, social insects, social cohesion, ants, Formica fusca
1. Introduction
A key challenge for evolutionary biology is to explain the emergence of cooperatively breeding groups, and identify the factors that influence the extent of helping in such groups (Seger 1993; Cahan et al. 2002). Cooperative breeding often entails unequal reproductive apportionment among group members (reproductive skew), such that some individuals monopolize breeding at the expense of the reproduction of others. A reduction in fitness incurred by curtailed personal reproduction can, however, be offset by indirect fitness returns when helpers are related to the reproductive individuals (Hamilton 1964). Higher inclusive fitness returns thus enhance social cohesion. Eusocial insects represent an example of extreme reproductive skew between colony queens and functionally sterile workers, where queens propagate genes to future generations and workers perform work that promotes colony growth. To add complexity, multiple reproductively active queens may be present in colonies of social insects (Hölldobler & Wilson 1977, 1990; Keller 1993), which raises the issue of reproductive apportionment among queens and so creates the potential for reproductive conflicts (Keller 1995). Such conflicts encompass both resident queens and workers, each having a vested interest in either personal reproduction or maximization of indirect fitness returns. In particular, workers may gain indirect fitness returns by favouring the queen to which they are most closely related.
When conflicts over offspring production emerge, they are unlikely to concern all types of brood equally. Thus, reproductive conflicts are likely to be rife over the production of sexual brood, but less so over the production of worker brood. Workers are an evolutionary dead end and, therefore, queens and workers alike are unlikely to gain a fitness benefit from promoting their genes through worker offspring. Indeed, in the event that there is a trade-off between worker and reproductive production by a queen, queens may be selected to minimize worker production (Reeve & Ratnieks 1993). Therefore, if queens in the colony differ in their level of reproductive dominance, the production of sexual offspring could be partitioned differently among new sexuals than among new worker offspring. At the extreme, such a secondary division of labour might evolve into social parasitism, where some queens gain sexual offspring but contribute nothing towards colony maintenance (Heinze & Keller 2000).
A few studies have examined the genetic congruence between worker and sexual offspring (Ross 1988, 1993; Pamilo & Seppä 1994; Heinze et al. 2001; Fournier et al. 2004; Kümmerli & Keller 2007). As predicted by the arguments earlier, most of these studies suggest that queens differ in their contribution to queen and worker brood (but see Heinze et al. 2001). However, no study so far has considered the interests of the workers to ask whether differences in queen contributions come at net benefit or cost to the inclusive fitness of the workers. We investigated reproductive partitioning among reproductive females (gynes) and workers in the ant Formica fusca and combined this information with data on the genetic returns gained by workers. We show that queens are unequally represented among gyne and worker brood. Furthermore, we show that this increases worker fitness relative to reproductive egalitarianism among queens. The non-random pattern of queen contribution to workers and gynes, therefore, raises the critical relatedness between workers and gynes, thereby promoting colony cohesion.
2. Material and methods
Our study species, Formica fusca (L), is a common soil-dwelling ant species which builds small colonies (500–2000 workers) in semi-open habitat (peat bogs, recently logged areas, etc.). The species is facultatively polygyne (i.e. colonies contain multiple queens), with previous estimates of effective queen number between two and five (Hannonen et al. 2004). Queens commence egg laying in spring, they continue laying for 2–3 weeks and this first offspring cohort comprises all sexual and most worker offspring to be produced during each breeding season. New workers are produced each year in discrete cohorts, and have an average lifespan of ca 1 year, i.e. workers born in July tend sexual brood the next spring and perish during their second summer. The study population, comprising around 60 colonies, is located on a 3 ha island, Lilla Träskön, off the coast of southern Finland. The population consists of both monogyne and polygyne colonies, with a yearly colony mortality of polygyne colonies of around 40% and an average rate of within-colony queen turnover close to 35% (Bargum et al. in press).
To obtain polygyne colonies, we collected adult workers from 56 colonies of unknown social structure. From as many colonies as possible, worker pupae (n=53), female sexual (gyne) pupae (n=32) and male pupae (n=29) were also collected on the same sampling occasion. As in other species of Formica, this species produces split sex ratios, with 59% of sexual-producing colonies producing only males or only gynes. In addition, we collected two to six established queens per colony from 13 polygyne colonies at the same site.
To determine the social type (monogyne or polygyne) of the colonies we had collected, we genotyped eight adult workers per colony at six polymorphic DNA-microsatellite loci (FL12, FL20: Chapuisat 1996; FE13, FE17, FE19, FE21: Gyllenstrand et al. 2002) following the protocol described in Hannonen et al. (2004). The colony-specific genotype distributions were inspected manually and the colonies were assigned as monogyne (n=18) if the genotypes were consistent with a single mother, or polygyne (n=38) if they were not. From 12 of the colonies determined as polygyne, sufficient numbers (n>10) of gyne and worker pupae were available for analysis. From these, we genotyped eight additional adult workers, up to 16 worker pupae, and up to 16 gyne pupae using the above protocol. The number of male-producing polygyne colonies with an adequate sample size was too small (n=5) to allow robust conclusions. Therefore, we restricted our analysis to colonies producing exclusively gynes. Finally, we dissected the established queens and genotyped them, as well as the contents of their spermatheca, again following the protocol described in Hannonen et al. (2004). Based on the genotype data thus obtained, we estimated relatedness with the program Relatedness v. 5.0.4 (Queller & Goodnight 1989). Average values were calculated by weighting nests equally, and standard errors were estimated by jackknifing over colonies.
We compared and quantified the number and identity of queens contributing to gyne and worker offspring in four steps. First, we compared the relatedness among gynes with that among worker brood within each colony. If the number of mothers and their reproductive apportionments are similar for both gynes and workers, the relatedness values should converge between the two types of offspring. If one caste is produced by fewer queens or skew is higher, we expect relatedness values to be higher within that caste.
Second, we quantified and compared the effective number of queens producing worker and gyne offspring using the formula
| (2.1) |
where rs is the average genetic relatedness among female offspring of a single queen (taking into account the frequency of multiple mating and the relatedness among the male mates of the queen); rQ is the average relatedness among nest mate queens; rm is the average relatedness among the males mated with different queens of the same colony (Queller 1993; Ross 1993; Seppä 1994); and rc is the estimated relatedness among offspring of one caste (e.g. Queller 1993; Ross 1993). To calculate rs, we estimated queen-mating frequency from the spermathecal contents obtained from the old queens. As we did not have access to offspring of these queens to estimate paternity skew and consequently effective mating frequency, we used the observed mating frequency. For the same reason, we could not estimate the relatedness between the male mates of queens and hence assumed rm to be zero, i.e. that the males were unrelated. This is a reasonable assumption as rm was shown to be zero in another population of the same age and species (Hannonen et al. 2004). These assumptions give a maximum estimate of effective queen number, which should be unbiased for the two offspring groups. RQ was estimated based on the genotypes of the established queens.
Third, to quantify the degree to which apportionment differs between worker and gyne brood, we estimated breeder shifting between castes (i.e. the degree to which the same queens contributed to gyne and worker brood, respectively) by applying the formula (Pedersen & Boomsma 1999)
| (2.2) |
where rg is the estimated relatedness among gyne offspring; rw is the relatedness among worker offspring; and rg,w is the relatedness between castes. This formula was originally developed to measure queen turnover, i.e. breeder shifting between temporally separated offspring clutches, but may equally well be used to describe differences in queen apportionment between any two groups. In our case, this represents a shift in breeder identity (breeder shifting) between different types of brood. The estimate can take values from 0, signifying no shift in the number and identity of queens producing gynes and workers, and 1, which signifies a complete absence of overlap in breeder identity between gyne and worker offspring. We also used this approach to compare changes in queen apportionment between the adult workers present in the colony and the gyne and worker brood they were raising. In this way, we can assess whether the same maternal queens that had produced the adult workers also produced the gynes and the new workers. To test for a difference in turnover between adult workers to gyne brood and adult workers to worker brood, we obtained confidence intervals for the turnover values by jackknifing over colonies.
Finally, we investigated whether the two offspring groups are genetically differentiated by partitioning the observed allelic diversity into variation between colonies, variation between castes within the same colony, and within-colony variation between individuals of the same caste, using the software Arlequin v. 2.0 (Schneider et al. 2000). The F-value of interest here is the one reflecting differentiation between castes within colonies. A significant deviation from zero would indicate that the castes are genetically differentiated. Such genetic differentiation may arise if the number of reproductive individuals producing each caste differs considerably, or if different individuals produce each caste. Since two colonies had missing data for one locus each, we calculated the F-values for two datasets: all 12 colonies using four loci and 10 colonies using all six loci. We also quantified the number of unique alleles, i.e. alleles found in one caste but not the other, within each colony across all loci.
To assess whether workers gain inclusive fitness benefits through a shift in the identity of queens producing worker and gyne brood, we estimated the relatedness of adult workers to gyne versus worker brood. We obtained the relatedness estimates by implementing the asymmetric relatedness algorithm (px–py) in Relatedness v. 5.0.4 (Queller & Goodnight 1989), assigning adult workers as px and either worker or gyne brood as py. We then conducted a pairwise comparison on the respective relatedness values to assess whether adult workers were more closely related to gynes than to worker brood.
3. Results
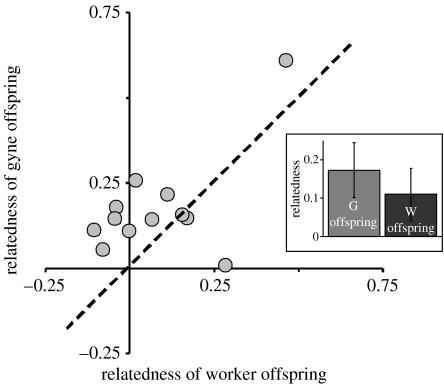
The relatedness among gyne offspring was consistently higher than that among worker offspring (gyne offspring r=0.17±0.07, worker offspring r=0.11±0.07, paired t-test: t=2.36, d.f.=11, two-tailed p=0.039; figure 1). These relatedness values translated into an effective number of queens of 14.2 for workers and 5.8 for gynes. Thus, either fewer queens produced gynes than workers, or at least the reproductive apportionment among them was much less equal for gyne than for worker production. For comparison, the average relatedness among adult workers was 0.14±0.07, intermediate to and statistically indistinguishable from that between gynes and worker brood. This estimate translates into an effective number of queens of 7.1.
Figure 1.
Relatedness among worker offspring plotted against relatedness among gyne offspring. The dashed line indicates where the points would fall if relatedness within the two offspring groups were equal. In most cases, gyne relatedness exceeds worker relatedness. Inset: bar chart of mean relatedness of worker and gyne offspring (±s.e.).
The magnitude of breeder shifting, i.e. the degree to which the reproductive apportionment of queens differs between worker and gyne brood, was significant (bs=0.71; t=4.32, d.f.=11, one-tailed p<0.001), indicating only a 29% overlap between the queens that produce gyne and worker brood. In agreement with this, gyne and worker brood were genetically differentiated (FST=0.019 for all colonies combined using four loci and FST=0.034 for 10 colonies combined using all six loci, p<0.001 in both cases). Unique alleles were present in both offspring groups; on average 2.09±0.48 and 2.73±0.63 for gyne and worker brood, respectively (mean±s.e.). Since the alleles present within gynes were not simply a subset of those appearing in the worker brood, this indicates that the difference in queen apportionment between gyne and worker brood is due to a shift in breeder identity, not only a change in skew.
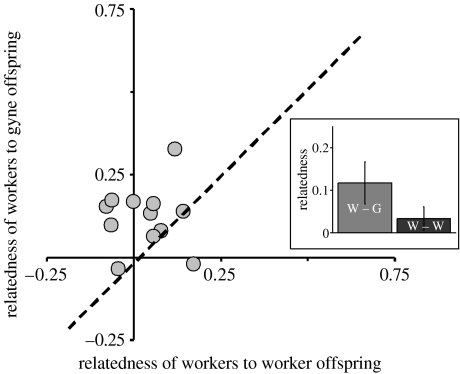
The estimated degree of breeder shifting was borderline significant between the cohort of adult workers and gynes (bs=0.28; t=1.80, d.f.=11, one-tailed p=0.051), and highly significant between adult workers and worker brood (bs=0.68; t=4.38, d.f.=11, one-tailed p<0.001). More importantly, breeder shifting between adult workers and gynes was significantly lower than that between adult workers and worker brood (t=123.41, d.f.=11, two-tailed p<0.001). This strongly suggests that to a large extent the same set of queens produced both the adult workers and the new gynes, whereas the set of queens producing new workers comprises different individuals. Indeed, in agreement with this, adult workers were significantly more related to gyne than to worker brood (mean relatedness to gynes r=0.12±0.05, to workers r=0.03±0.03, t=2.37, d.f.=11, one-tailed p=0.019, figure 2).
Figure 2.
Relatedness between workers and the two offspring groups. The dashed line indicates where points would fall if workers were equally related to the two offspring groups. In most cases, workers are more closely related to gyne than to worker offspring. Inset: bar chart of mean relatedness of workers to worker and gyne offspring (±s.e.).
4. Discussion
Here, we provide the first evidence that differential reproductive partitioning among breeders can enhance the inclusive fitness returns for sterile individuals that tend non-descendant offspring. Two aspects of reproductive partitioning contribute to this outcome. First, the effective number of queens contributing to gyne (new reproductive female) brood is significantly lower than that contributing to worker brood, such that relatedness increases from worker to gyne brood. Second, and more importantly, adult workers were significantly more related to the reproductive brood raised by the colony, than to the contemporary worker brood. Thus, the observed breeder shift leads to genetic benefits for the adult workers that tend the brood. Our results also have repercussions for genetic population analyses. Given the observed pattern of reproductive partitioning, estimates of effective population size based on worker and gyne samples are not interchangeable.
A difference in the number of queens producing each caste may arise if a subset of the available mother queens produce gynes (i.e. skew is higher in gyne production than worker production; Ross 1993). Alternatively, at least partially different sets of mother queens may produce gynes and workers (Ross 1988; Fournier et al. 2004; Kümmerli & Keller 2007). In our case, both these scenarios apply. The fact that contemporary gyne and worker brood were genetically distinct strongly suggests that at least partially different sets of queens contribute to gyne and worker brood. This implies considerable fitness differences between queens, unless breeders are exchanged between seasons and young queens produce workers while queuing for the opportunity to produce gynes. Nevertheless, fewer queens produced gynes than worker offspring, which suggests that only a subset of all queens come to produce gynes. The study colonies included here produced exclusively gynes, so these results cannot be explained by queens specializing in producing either male or female sexuals (Fournier & Keller 2001; Sumner et al. 2001; Fournier et al. 2004). At present, the issue of reproductive partitioning between worker and male brood remains open, in particular as the comparison of the parameters used here between diploid and haploid brood are anything but straightforward.
Arguably, the fact that fewer queens contribute to gyne production than worker production would imply that workers trade a genetic gain in 1 year to a loss in the next, because relatedness in the new cohort of workers, being produced by a greater number of queens, will be lower. However, the average relatedness among adult workers (r=0.14) was close to that among worker brood (r=0.11), although the set of queens that produced the two cohorts may be largely non-overlapping. Thus, the new workers are in a position to favour their mothers, and the same opportunity for enhanced inclusive fitness returns is likely to be available also for these workers. Indeed, the yearly colony mortality of 40% and a rate of queen turnover of 35% (Bargum et al. in press) suggest that such an opening is likely to be available for each new worker cohort.
The conclusion that queens contribute unequally to gyne production and that workers consequently gain an inclusive fitness increment, begs the question of whether the observed pattern is driven by queen turnover (exchange of breeders) between years, or whether active intervention by workers is involved. The observed pattern may arise without active intervention by the workers if, for example, the queens that produced gynes in the study year also were the mothers of the adult workers, and the queens that produced the new workers were the sisters of the adult workers. Thus, young queens may produce workers in their first year(s) of breeding and produce gynes in later years. This would imply extensive queen turnover between years in this species on one hand, and provide a mechanism by which queens cannot parasitize on colony resources by producing exclusively gynes on the other. Three lines of evidence are consistent with this. In a study across four consecutive worker cohorts, we have shown that there is extensive queen turnover (approx. 35%) between years (Bargum et al. in press). In addition, pilot studies show that young (first-year breeders) tend to start laying eggs slightly later in the season and that eggs laid towards the end of this initial egg-laying period are more likely to develop into workers than gynes (K. Bargum, R. Ovaska 2004, 2005, personal observation).
Alternatively, active intervention by workers may be involved and could arise in two ways. First, if workers preferentially feed close relatives among larvae, these may be more likely to enter the queen development pathway than the worker pathway. In contrast to sex ratio manipulation (Reuter et al. 2004), this manipulation could occur at negligible costs to the colony, unless an excess of queens is produced (Reuter et al. 2004; Wenseleers & Ratnieks 2004). In F. fusca, there is indeed evidence that workers recognize and favour their relatives during brood development (Hannonen & Sundström 2004). Second, workers could influence which queens survive and/or come to breed in the season of their own adulthood, and in so doing favour close relatives or disfavour more distant relatives. The fitness benefit gained from manipulation will be transient, because the next cohort of adult workers will, in turn, favour their relatives, but are less related to the current cohort of adult workers. This creates an incentive for workers to prevent queens of lower kin value from also producing worker offspring. However, colony level costs in terms of productivity may prohibit this. In addition, given the short lifespan of colonies, selection for actions with such long-term effects may be weak. At present, however, the question whether worker intervention is involved or not remains unresolved.
In conclusion, regardless of the underlying mechanisms, our results show that queens share reproduction more unequally with respect to gyne production than worker production, and, more importantly, that queen shifts occurs in a direction that benefits the workers. As a result, the breeding system can come to enhance the inclusive fitness of the workers and thus promote social cohesion also in the presence of multiple queens. Only a few studies have previously shown that breeding systems with multiple breeders may serve the interests of the collective. In polistine wasps and the ant Formica exsecta, replacement queens are produced only when queen numbers are very low, leading to high relatedness between co-breeding queens (Queller et al. 1993; Brown & Keller 2000; Tsuchida et al. 2000; Henshaw et al. 2004). However, the pattern observed in this study perhaps more closely resembles that found in groups of white-winged choughs, where individuals with a consort of relatives more often obtain dominance status than single individuals (Heinsohn et al. 2000). Thus, by serving kin interests, breeding systems including multiple breeders may nevertheless enhance social cohesion.
Acknowledgments
We would like to dedicate this study to the memory of Rainer Rosengren who was instrumental at the early stages of this study. We also thank S. Kupiainen and M. Rehn for their lab assistance, and Kevin Foster and Chris DeHeer for their comments. K.B. was supported by the Finnish School in Wildlife Biology, Conservation and Management and by grants from the Waldemar von Frenckell and Otto A. Malm foundations. L.S. was supported by research grants from the Academy of Finland (42725 and 206505) and the Finnish Society for Sciences and Letters.
References
- Bargum, K., Helanterä, H., & Sundström, L. In press. Genetic population structure, queen supersedure and social polymorphism in a social Hymenoptera. J. Evol. Biol [DOI] [PubMed]
- Brown W.D, Keller L. Colony sex ratios vary with queen number but not relatedness asymmetry in the ant Formica exsecta. Proc. R. Soc. B. 2000;267:1751–1757. doi: 10.1098/rspb.2000.1206. doi:10.1098/rspb.2000.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahan S.H, Blumstein D.T, Sundström L, Liebig J, Griffin A. Social trajectories and the evolution of social behavior. Oikos. 2002;96:206–216. doi:10.1034/j.1600-0706.2002.960202.x [Google Scholar]
- Chapuisat M. Characterization of microsatellite loci in Formica lugubris B and their variability in other ant species. Mol. Ecol. 1996;5:599–601. doi: 10.1111/j.1365-294x.1996.tb00354.x. doi:10.1046/j.1365-294X.1996.00124.x [DOI] [PubMed] [Google Scholar]
- Fournier D, Keller L. Partitioning of reproduction among queens in the Argentine ant, Linepithema humile. Anim. Behav. 2001;62:1039–1045. doi:10.1006/anbe.2001.1848 [Google Scholar]
- Fournier D, Aron S, Keller L. Significant reproductive skew in the facultatively polygynous ant Pheidole pallidula. Mol. Ecol. 2004;13:203–210. doi: 10.1046/j.1365-294x.2003.02036.x. doi:10.1046/j.1365-294X.2003.02036.x [DOI] [PubMed] [Google Scholar]
- Gyllenstrand N, Gertsch P.J, Pamilo P. Polymorphic microsatellite DNA markers in the ant Formica exsecta. Mol. Ecol. Notes. 2002;2:67–69. doi:10.1046/j.1471-8286.2002.00152.x [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. I and II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hannonen M, Sundström L. Worker nepotism among polygynous ants. Nature. 2004;421:910. doi: 10.1038/421910a. doi:10.1038/421910a [DOI] [PubMed] [Google Scholar]
- Hannonen M, Helanterä H, Sundström L. Habitat age, breeding system and kinship in the ant Formica fusca. Mol. Ecol. 2004;13:1579–1588. doi: 10.1111/j.1365-294X.2004.02136.x. doi:10.1111/j.1365-294X.2004.02136.x [DOI] [PubMed] [Google Scholar]
- Heinsohn R, Dunn P, Legge S, Double M. Coalitions of relatives and reproductive skew in cooperatively breeding white-winged choughs. Proc. R. Soc. B. 2000;267:243–249. doi: 10.1098/rspb.2000.0993. doi:10.1098/rspb.2000.0993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze J, Keller L. Alternative reproductive strategies: a queen perspective in ants. Trends Ecol. Evol. 2000;15:508–512. doi: 10.1016/s0169-5347(00)01995-9. doi:10.1016/S0169-5347(00)01995-9 [DOI] [PubMed] [Google Scholar]
- Heinze J, Trunzer B, Hölldobler B, Delabie J.H.C. Reproductive skew and queen relatedness in an ant with primary polygyny. Insect. Soc. 2001;48:149–153. doi:10.1007/PL00001758 [Google Scholar]
- Henshaw M.T, Robson S.K.A, Crozier R.H. Queen number, queen cycling and queen loss: the evolution of complex multiple queen societies in the social wasp genus Ropalidia. Behav. Ecol. Sociobiol. 2004;55:469–476. doi:10.1007/s00265-003-0725-x [Google Scholar]
- Hölldobler B, Wilson E.O. The number of queens: an important trait in ant evolution. Naturwissenschaften. 1977;64:8–15. doi:10.1007/BF00439886 [Google Scholar]
- Hölldobler B, Wilson E.O. Belknap University Press; Cambridge, UK: 1990. The ants. [Google Scholar]
- Keller L, editor. Queen number and sociality in insects. Oxford University Press; Oxford, UK: 1993. [Google Scholar]
- Keller L. Social life: the paradox of multiple-queen colonies. Trends Ecol. Evol. 1995;10:355–360. doi: 10.1016/s0169-5347(00)89133-8. doi:10.1016/S0169-5347(00)89133-8 [DOI] [PubMed] [Google Scholar]
- Kümmerli R, Keller L. Reproductive specialization in multiple-queen colonies of the ant Formica exsecta. Behav. Ecol. 2007;18:375–383. doi:10.1093/beheco/arl088 [Google Scholar]
- Pamilo P, Seppä P. Reproductive competition and conflicts in colonies of the ant Formica sanguinea. Anim. Behav. 1994;48:1201–1206. doi:10.1006/anbe.1994.1352 [Google Scholar]
- Pedersen J.S, Boomsma J.J. Effect of habitat saturation on the number and turnover of queens in the polygynous ant, Myrmica sulcinodis. J. Evol. Biol. 1999;12:903–917. doi:10.1046/j.1420-9101.1999.00109.x [Google Scholar]
- Queller D.C. Genetic relatedness and its components in polygynous colonies of social insects. In: Keller L, editor. Queen number and sociality in insects. Oxford University Press; Oxford, UK: 1993. pp. 132–152. [Google Scholar]
- Queller D.C, Goodnight K.F. Estimation of genetic relatedness using allozyme data. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Strassmann J.E, Solis C.R, Hughes C.R, Deloach D.M. A selfish strategy of social insect workers that promotes social cohesion. Nature. 1993;365:639–641. doi:10.1038/365639a0 [Google Scholar]
- Reeve K.R, Ratnieks F. Queen–queen conflicts in polygynous societies: mutual tolerance and reproductive skew. In: Keller L, editor. Queen number and sociality in insects. Oxford University Press; Oxford, UK: 1993. pp. 45–85. [Google Scholar]
- Reuter M, Helms K.R, Lehmann L, Keller L. Effects of brood manipulation costs on optimal sex allocation in social Hymenoptera. Am. Nat. 2004;164:E73–E82. doi: 10.1086/422659. doi:10.1086/422659 [DOI] [PubMed] [Google Scholar]
- Ross K.G. Differential reproduction in multiple-queen colonies of the fire ant Solenopsis invicta (Hymenoptera: Formicidae) Behav. Ecol. Sociobiol. 1988;23:341–355. doi:10.1007/BF00303708 [Google Scholar]
- Ross K.G. The breeding system of the fire ant Solenopsis invicta: effects on colony genetic structure. Am. Nat. 1993;141:554–576. doi: 10.1086/285491. doi:10.1086/285491 [DOI] [PubMed] [Google Scholar]
- Schneider S, Roessli D, Excofficier L. Genetics and Biometry Laboratory, University of Geneva; Geneva, Switzerland: 2000. Arlequin ver. 2.000: a software for population genetic analysis. [Google Scholar]
- Seger J. Opportunities and pitfalls in co-operative reproduction. In: Keller L, editor. Queen number and sociality in insects. Oxford University Press; Oxford, UK: 1993. pp. 1–16. [Google Scholar]
- Seppä P. Sociogenetic organization of the ants Myrmica ruginodis and Myrmica lobicornis—number, relatedness and longevity of reproducing individuals. J. Evol. Biol. 1994;7:71–95. doi:10.1046/j.1420-9101.1994.7010071.x [Google Scholar]
- Sumner S, Casiraghi M, Foster W, Field J. High reproductive skew in tropical hover wasps. Proc. R. Soc. B. 2001;269:179–186. doi: 10.1098/rspb.2001.1884. doi:10.1098/rspb.2001.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K, Ito Y, Katada S, Kojima J. Genetical and morphological colony structure of the Australian swarmfounding polistine wasp, Ropalidia romandi (Hymenoptera, Vespidae) Insectes Soc. 2000;47:113–116. doi:10.1007/PL00001688 [Google Scholar]
- Wenseleers T, Ratnieks F.L.W. Tragedy of the Commons in Melipona bees. Proc. R. Soc. B. 2004;271:S310–S312. doi: 10.1098/rsbl.2003.0159. doi:10.1098/rsbl.2003.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]