Abstract
How the radial body plan of echinoderms is related to the bilateral body plan of their deuterostome relatives, the hemichordates and the chordates, has been a long-standing problem. Now, using direct development in a sea urchin, I show that the first radially arranged structures, the five primary podia, form from a dorsal and a ventral hydrocoele at the oral end of the archenteron. There is a bilateral plane of symmetry through the podia, the mouth, the archenteron and the blastopore. This adult bilateral plane is thus homologous with the bilateral plane of bilateral metazoans and a relationship between the radial and bilateral body plans is identified. I conclude that echinoderms retain and use the bilateral patterning genes of the common deuterostome ancestor. Homologies with the early echinoderms of the Cambrian era and between the dorsal hydrocoele, the chordate notochord and the proboscis coelom of hemichordates become evident.
Keywords: pentamery, morphogenesis, evolution, Holopneustes purpurescens, echinoid, irregular sea urchins
1. Introduction
The echinoderms have a radially arranged, pentamerous body structure that is very different from the bilateral body structure of the related deuterostome phyla, the hemichordates and the chordates. Yet, all three phyla evolved from a common bilaterally symmetric ancestor (Eernisse & Peterson 2004; Smith et al. 2004). The way structures changed in form during the evolution of the radial echinoderms and which structures are homologous between the phyla are still problematic (Smith et al. 2004). Some solutions were found here by investigating the embryonic origins of the first radially arranged structures, the five primary podia, in a sea urchin, Holopneustes purpurescens, that develops the adult echinoderm structures directly (Morris 1995) without a feeding larval stage. The five primary podia head the echinoid growth zones from which grow the five ambulacra of the adult echinoderm (Mooi et al. 1994). Using H. purpurescens, the morphogenesis of the adult echinoderm can be observed during the very early stages of development.
The embryonic origins of the primary podia were investigated here morphologically using the histological technique of serial sectioning. This method contrasts with the molecular approaches that have used gene expression studies in developing sea urchins to address problems relating to the origins of the echinoderm radial body plan (Arenas-Mena et al. 2000; Peterson et al. 2000; Morris & Byrne 2005).
2. Material and methods
Vestibula larvae of H. purpurescens were cultured as described (Morris & Byrne 2005). Larval stages from 25 to 34 h post-fertilization, sampled at 1 h intervals, were fixed in 4% paraformaldehyde (Sigma–Aldrich Co.) in filtered seawater for 2 h, dehydrated in an ethanol series and embedded in Procure 812 resin (ProSciTech Pty). Serial sections were cut at 10 μm and mounted in DPX (ProSciTech Pty). The larval stages were sectioned in the frontal and sagittal planes, and some were sectioned in the transverse plane. All stages were examined but only selected serial sections from 29 and 34 h larvae are shown here. Larvae of 44 h were prepared as whole mounts as described (Morris & Byrne 2005). Sections, under phase contrast optics, and whole mounts were viewed and photographed as described (Morris & Byrne 2005) or were recorded digitally.
3. Results
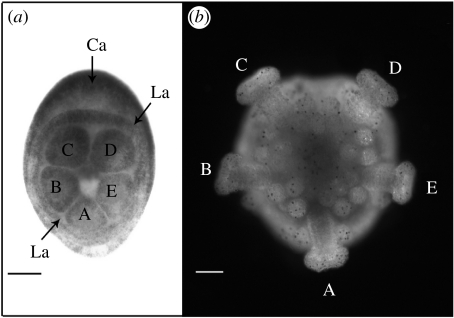
Holopneustes purpurescens develops through a non-feeding vestibula larva (figure 1a), metamorphosing into a juvenile sea urchin (figure 1b) within a few days of fertilization (Morris 1995). The five primary podia (figure 1), which are the earliest structures to show the pentamery that characterizes the echinoderm body plan, are well developed in a vestibula larva of 44 h (figure 1a). They are named from A to E (figure 1), using the Carpenter labels (Hyman 1955), based on the position of genital plate 2 and the hydropore between podia C and D (Morris 1995). The way in which these podia develop in H. purpurescens was investigated in a range of larval stages that were fixed for histology and serially sectioned. The development is described here from selected serial sections of 29 and 34 h larvae.
Figure 1.
The five primary podia of H. purpurescens. The podia are named A–E, using the Carpenter system (Hyman 1955). (a) Vestibula larva of 44 h (oral view), blastopore (now closed) towards the base of the panel. The Carpenter axis (Ca) is between podia C and D and through podium A. Lovén's axis (La) is through podium D and between podia B and A. (b) Juvenile sea urchin of 8 days (oral view), same orientation as in (a). Scale bar, 100 μm.
(a) The dorsal and ventral hydrocoeles
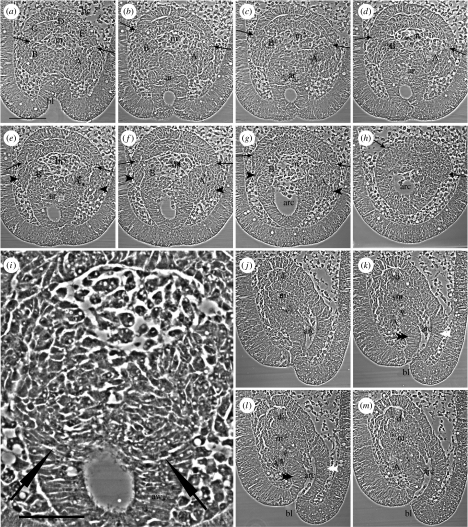
The development of the five primary podia from two hydrocoeles is shown first in a 29 h larva sectioned in the frontal plane, parallel to the oral face of the larva (figure 2a–h). The sections (figure 2a–h) are in oral to aboral order, oriented with the blastopore towards the base of each panel. The first section (figure 2a) shows, within the blastocoele, the hydrocoele cells at the inner end of the archenteron. Two hydrocoeles are identified, an upper, dorsal hydrocoele and a lower, ventral hydrocoele. The division between the dorsal and ventral hydrocoeles is marked by a pair of arrows in each panel (figure 2a–h): the hydrocoeles are well separated at their lateral edges in the early sections (e.g. figure 2a). Between the two hydrocoeles is the mouth cavity (figure 2a–g). The five primary podia will form at the oral ends of the dorsal and ventral hydrocoeles. To be noted is the origin of podia C, D and E from the dorsal hydrocoele and of podia B and A from the ventral hydrocoele (figure 2a).
Figure 2.
Selected serial sections through vestibula larvae of 29 h (described in text). (a–h) Sections cut in the frontal plane, in oral to aboral order (oral view). A pair of arrows in each panel (a–h) marks the division between the dorsal and ventral hydrocoeles. Sections (b–f) are successive serial sections. (i) Enlargement of part of (e); tips of fine arrows mark the transition between cells of the archenteron wall (aw) and cells of the oral wall of the archenteron that contribute to the ventral hydrocoele. (j–m) Sections cut in the sagittal plane (oral left, aboral right); they are successive serial sections. A–E, primary podia anlagen; ar, archenteron; arc, archenteron cavity; bc, blastocoele; bl, blastopore; d, dorsal hydrocoele; m, mouth; v, ventral hydrocoele; black arrowhead, wings at start of B or A lobes; double black arrowhead, oral wall of archenteron; double white arrowhead, aboral wall of archenteron. Scale bars, (a) 100 μm and (i) 50 μm.
The dorsal and ventral hydrocoeles, when traced through later sections (figure 2b–h), join with parts of the archenteron wall. Tracing the division between the hydrocoeles, marked by the pairs of arrows, shows that the dorsal hydrocoele joins with the upper, aboral wall of the archenteron (figure 2b–h). Tracing the ventral hydrocoele to the archenteron wall is more complex. In the early sections (e.g. figure 2a), the ventral hydrocoele from which podia B and A form is a continuous lobe. In later sections (e.g. figure 2b), the continuous lobe separates into two lobes, the B lobe and the A lobe. The narrow junction between the B and A lobes (figure 2b) then merges with the oral wall of the archenteron (figure 2c–e). The remnants of the lobes then join with the lateral walls of the archenteron (figure 2f,g). An enlargement (figure 2i) from figure 2e shows the transition (fine arrows, figure 2i) from cells of the archenteron wall (aw, figure 2i) to cells of the oral wall of the archenteron that contribute to the ventral hydrocoele. That the B and A lobes overhang the oral wall of the archenteron is evidenced by the wings at the sides of the lateral walls of the archenteron (figure 2e–g): the wings are the beginnings of the B and A lobes. Thus, the B lobe joins with the right lateral archenteron wall of the larva and the A lobe joins with the left lateral archenteron wall of the larva. The connexion of the mouth cavity with the cavity of the archenteron is evident in the later sections (figure 2f,g).
Sagittal sections of a 29 h larva (figure 2j–m) confirm the anatomy described for the frontal sections. The dorsal and ventral hydrocoeles are separated by the mouth cavity (figure 2j–m) and this connects with the cavity of the archenteron, which opens at the blastopore (figure 2k–m). The dorsal and ventral hydrocoeles connect with the aboral and oral walls of the archenteron, respectively (figure 2k,l). The lobe of the ventral hydrocoele, which is here lobe A, overhangs the oral wall of the archenteron (figure 2k–m).
(b) Podia C, D and E and podia B and A form from different hydrocoeles
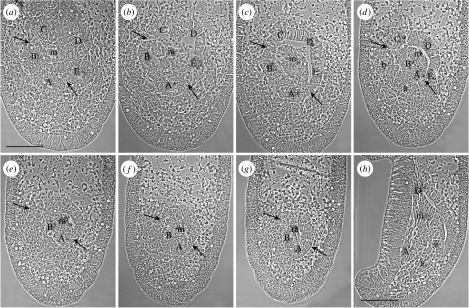
A later stage of development of the five primary podia in a 34 h larva, when the podial termini can be identified, shows the origin of podia C, D and E from the dorsal hydrocoele and podia B and A from the ventral hydrocoele. The sections shown (figure 3a–g) are in the frontal plane in oral to aboral order in the same orientation as in figure 2. The first section (figure 3a) shows the five primary podial termini which are the oral ends of the primary podia. The podia at this stage have rotated as a group in a clockwise direction from their origins in the 29 h larva (figure 2a), such that podium A now occupies a position similar to that in the 44 h larva (figure 1a). The division between the dorsal and ventral hydrocoeles from which the podia form is again marked by pairs of arrows in each panel (figure 3a–g). In the first two sections (figure 3a,b), the arrows are between podia C and B and between podia E and A. When these sites are traced to later sections, they lie over the division between the dorsal and ventral hydrocoeles (figure 3c–e). A connexion between the coelomic cavities of the dorsal and ventral hydrocoeles at a level just aboral to the podia (figure 3c) cannot be discounted. The mouth cavity is inside the ring of podial termini in the first sections (figure 3a,b). In later sections (e.g. figure 3c), the mouth cavity is between the two hydrocoeles.
Figure 3.
Selected serial sections through vestibula larvae of 34 h (described in text). (a–g) Sections cut in the frontal plane, in oral to aboral order (oral view). A pair of arrows in each panel (a–g) marks the division between the dorsal and ventral hydrocoeles. (h) A section in the sagittal plane (oral left, aboral right). A–E, primary podia anlagen; a, loosely structured cells of the A lobe; b, loosely structured cells of the B lobe; m, mouth; s, somatocoele cells; v, ventral hydrocoele wall. Scale bar, 100 μm.
The connexions of the podia to the archenteron wall in the 34 h larva are traced by following the outer epithelium of each podial terminus aborally through the series of sections (figure 3b–g). The outer epithelia of podia C, D and E connect with the upper, aboral wall of the archenteron (figure 3b–g). The outer epithelium of podium B joins with the right lateral archenteron wall of the larva (figure 3b–g). The outer epithelium of podium A joins more with the oral wall of the archenteron (figure 3b–f). It does not obviously join the left lateral archenteron wall of the larva, as the A lobe does in the 29 h larva, but the division between the two hydrocoeles (figure 3e, right arrow) is traced to the left lateral archenteron wall of the larva (figure 3g, right arrow). In the 34 h larva, the archenteron has shortened and separated from its earlier connexion with the blastopore, which is now closed.
Peripheral to the structured outer epithelia of the B and A podia are less well-structured groups of cells that resemble the B and A lobes of the ventral hydrocoele in the 29 h larva (figure 3d, compare with figure 2a–d). These groups of cells sit aborally beneath the profiles of the B and A podial termini in the early sections (figure 3d, compare with figure 3a). They contribute to the morphogenesis of the B and A podia possibly by interdigitation, forming an epithelium. The evidence from the sections of the 34 h larva taken together (figure 3) suggests that the B and A podia stem from the lateral and oral archenteron walls and that the loosely structured B and A cells and the structured epithelia of the B and A podia grow across the frontal plane towards the larval right, and rotate clockwise. There is evidence in the 29 h larva of the start of this morphogenetic growth, which is illustrated in an interpretive diagram of the origin of the podia (figure 4). In sagittal sections, mesodermal cells that possibly form the somatocoeles seem to come from the oral wall of the archenteron below the origin of the ventral hydrocoele (figure 3h).
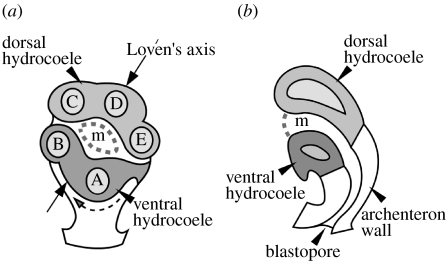
Figure 4.
Diagram of the origin of the five primary podia in the vestibula larva. (a) Frontal (oral) view turned slightly to the larval right. Podia C, D and E form from the dorsal hydrocoele and podia B and A form from the ventral hydrocoele. Dashed curved arrow shows the direction of morphogenetic rotation. (b) Sagittal view through the dorsal and ventral hydrocoeles, the mouth, the archenteron and the blastopore. A–E, the primary podia; m, adult mouth.
4. Discussion
These results show that the H. purpurescens vestibula larva has a bilateral structure. There is a plane of bilateral symmetry through the mouth, the archenteron and the blastopore. At the level of the five podia, this bilateral plane passes through podium D and between podia B and A, on the evidence that podia B and A form from the right and left lateral walls of the archenteron in the 29 h larva. This bilateral plane through the podia is coincident with Lovén's axis, which is one of the three bilateral axes used in descriptions of the echinoderm radial plan (Hyman 1955; David et al. 1995). In older larvae, however, morphogenetic growth has led to a small clockwise rotation of Lovén's axis in frontal view such that the bilateral plane of the larva appears to be coincident with the Carpenter axis (figure 1a).
The bilateral plane of the H. purpurescens vestibula larva is homologous, I suggest, with the bilateral plane of the bilateral phyla because it is through the same structures, namely, the adult mouth, the archenteron and the blastopore, as the bilateral plane is in the bilateral phyla. The anterior–posterior axis of the vestibula larva is then from the mouth to the blastopore with the mouth anterior and the blastopore posterior. The five podia form around the anterior end of this axis, and to this extent allow the five podia to be thought of as lateral outgrowths from an anterior–posterior axis, as concluded by David & Mooi (1996) and Peterson et al. (2000). The podia, however, form from the archenteron wall as a group of three plus two, a group that has bilateral symmetry so that a further axis, a dorsal–ventral axis, needs to be specified. Here, the dorsal–ventral axis is set through the aboral–oral axis and its polarity is based on the epithelial structure of the archenteron wall: on the aboral side, named as dorsal, the epithelium is intact with no de-epithelialization, whereas on the oral side, named as ventral, the epithelium shows de-epithelialization with mesodermal cells near by in the blastocoelar space (figure 3h). The sides are named as dorsal and ventral because these epithelia resemble those of the dorsal and ventral archenteron walls in a vertebrate embryo (Gilbert 1988). The hydrocoeles and podia that develop from the respective aboral and oral archenteron walls are therefore referred to as dorsal and ventral hydrocoeles or dorsal and ventral sets of podia. With respect to the polarity of the mouth in the vestibula larva, the bending of the anterior region away from a linear axis (figure 2j–m) moves the mouth and the hydrocoeles through about 90°, creating the echinoderm oral side. The mouth thus has both an anterior and a ventral polarity, as it does in vertebrates. If the results reported for the H. purpurescens vestibula larva were present in other echinoderms, the axes and polarities named here would be those of the echinoderm body plan.
Evidence that the plane of bilateral symmetry and the dorsal and ventral hydrocoeles reported for the vestibula larva of H. purpurescens are present in other echinoderms comes first from the irregular echinoids. The bilateral plane in the vestibula larva of H. purpurescens before the rotation that occurs between 29 and 34 h is through Lovén's axis. This is the plane along which a tendency of the periproct to retreat out of the apical system occurred in eight independent echinoid lineages, succeeding fully in only one, the lineage leading to the irregular echinoids (Saucède et al. 2003). This suggests that the bilateral plane of the vestibula larva is present in all echinoids, regular and irregular, making it general for one class of echinoderms. Also, if the dorsal hydrocoele from which three podia form and the ventral hydrocoele from which two podia form, described for the vestibula larva, were present in the abyssal irregular pourtalesiids, it would explain the unusual arrangement of their ocular plates which are separated into an anterior group of three plates and a posterior group of two (Saucède et al. 2004). There is thus support for supposing that structures described for the vestibula larva apply to regular and irregular echinoids, making it unlikely that the condition in H. purpurescens is highly derived and unrepresentative of a larger group.
Evidence that the embryonic bilateral symmetry of the vestibula larva of H. purpurescens might be general for echinoderms comes from Camptostroma, a Lower Cambrian echinoderm near the base of the echinoderm radiation (Paul & Smith 1984). The bilateral symmetry of the vestibula larva through the podia arranged as a dorsal group of three and a ventral group of two has resemblance to the ambulacral arrangement in Camptostroma, in which there is bilateral symmetry through the central ambulacrum of the three directed away from the periproct and the periproct itself, with the other two ambulacra directed oppositely on either side of the periproct (Paul & Smith 1984).
Further evidence for generality can be found in theories of the origin of the pentaradiate condition from the triradiate condition (see discussion in Hyman 1955, p. 694). The helicoplacoids of the Lower Cambrian were triradiate (Paul & Smith 1984). The triradiate condition would be explained if the dorsal hydrocoele formed only a single podium and ambulacrum. The pentaradiate condition, I hypothesize, would have evolved from the development of two further podia from the dorsal hydrocoele on either side of the single podium. This interpretation is in agreement with that given by Paul & Smith (1984) for the helicoplacoids in so far as they describe a single ambulacrum opposite the pair of ambulacra. They describe the pentaradiate condition, however, in agreement with Bather (1900), as arising from a branching of each member of the pair of ambulacra into two, resulting in a 2+1+2 pattern of ambulacra applicable to all pentaradiate echinoderms. The embryological evidence I report here shows that the pattern might also be described as a 1+3+1 pattern from the arrangement of the dorsal and ventral hydrocoeles and the podia formed from them. This difference in the two descriptions is of less significance than the agreement between them, namely, that there is a line of bilateral symmetry through the central ambulacrum of the 2+1+2 and the 1+3+1 patterns and each periproct. I have not attached Carpenter labels to the ambulacra in these patterns because the Carpenter labels, specified in relation to the position of the madreporite (Hyman 1955), will not be the same for possible homologous ambulacra in forms where the periproct and madreporite are not in the same interambulacrum, as they are not in echinoids, and where they are in the same interambulacrum, as they are in edrioasteroids (Paul & Smith 1984; electronic supplementary material). The difference between echinoids and forms such as edrioasteroids in the location of the madreporite, however, requires explanation.
The results reported here have depended on the mode of development of H. purpurescens, wherein the adult echinoderm structures form in a continuous morphogenetic process not dissociated from gastrulation. Early in this process, the dorsal and ventral hydrocoeles form at the head of the archenteron from different parts of the archenteron wall and they are structurally different. Only later, when the podia form, is there evidence that the cavities of the hydrocoeles become confluent. This early development would not be easy to observe in sea urchin species that develop through a feeding pluteus larva since, in these species, the primary podia at the end of an extended axocoele lack a clear connexion with the archenteron wall (von Ubisch 1913). With no evidence in the vestibula larva of the right-side coeloms of the pluteus larva, the hydrocoeles in the vestibula larva are equivalent to the left hydrocoele in the pluteus larva. In vestibula larvae older than those shown here, the dorsal hydrocoele develops a connexion to the exterior at the hydropore from the region of the dorsal hydrocoele that forms podium D. There is no evidence, other than this, of an axocoele. So, structures seen in the pluteus larva are absent in the vestibula larva. These absences are supportive of recent ideas (Swalla 2006) that feeding larvae evolved secondarily during echinoderm evolution. The closest resemblances to the embryonic structures in the vestibula larva are those in holothurians (Ohshima 1921; Runnström 1927), where a hydrocoele forms at the head of the archenteron and where the hydrocoele is not separated into another more anterior coelom, the axocoele (Smiley 1986).
The axes and polarities of the echinoderm body plan proposed here and the identification of a dorsal hydrocoele invite speculation on morphological homologies between the radial echinoderms and the related bilateral phyla (Smith et al. 2004), the chordates and hemichordates. The similarity between the origin of the dorsal hydrocoele from the dorsal (aboral) wall of the archenteron and the origin of the notochord in chordates from the dorsal wall of the archenteron (Gilbert 1988) points to a possible homology between these structures. In hemichordates, the candidate homologous structure would be the coelom of the proboscis (Hyman 1959). This coelom opens via a dorsal hydropore (Hyman 1959), a structure that in older vestibula larvae connects with the dorsal hydrocoele region of podium D.
The identification in an echinoderm of a bilateral plane homologous with that of its deuterostome relatives leads to the conclusion that the genes that patterned the bilateral form in the deuterostome ancestor are still active in extant echinoderms.
The discovery of embryonic dorsal and ventral hydrocoeles, and the development of the primary podia from them, contributes new data that will impact on the interpretation of embryonic and larval structures in the other classes of echinoderms and the lines of echinoderm evolutionary descent.
Supplementary Material
Differences in the labelling of the 2+1+1 and the 1+3+1 ambulacral patterns are explained
References
- Arenas-Mena C, Cameron A.R, Davidson E.H. Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development. 2000;127:4631–4643. doi: 10.1242/dev.127.21.4631. [DOI] [PubMed] [Google Scholar]
- Bather F.A. The echinoderma. In: Lankester E.R, editor. A treatise on zoology, part III. Adam & Charles Black; London, UK: 1900. pp. 1–344. [Google Scholar]
- David B, Mooi R. Embryology supports a new theory of skeletal homologies for the phylum Echinodermata. C. R. Acad. Sci. Paris. 1996;319:577–584. [Google Scholar]
- David B, Mooi R, Telford M. The ontogenetic basis of Lovén's rule clarifies homologies of the echinoid peristome. In: Emson R, Smith A, Campbell A, editors. Echinoderm research 1995. Balkema; Rotterdam, The Netherlands: 1995. pp. 155–164. [Google Scholar]
- Eernisse D.J, Peterson K.J. The history of animals. In: Cracraft J, Donoghue M.J, editors. Assembling the tree of life. Oxford University Press; New York, NY: 2004. pp. 197–208. [Google Scholar]
- Gilbert S.F. 2nd edn. Sinauer; Sunderland, MA: 1988. Developmental biology. [Google Scholar]
- Hyman L.H. The invertebrates: Echinodermata. vol. IV. McGraw-Hill; New York, NY: 1955. [Google Scholar]
- Hyman L.H. The invertebrates: smaller coelomate groups. vol. V. McGraw-Hill; New York, NY: 1959. [Google Scholar]
- Mooi R, David B, Marchand D. Echinoderm skeletal homologies: classical morphology meets modern phylogenetics. In: David B, Guille A, Féral J.P, Roux M, editors. Echinoderms through time. Balkema; Rotterdam, The Netherlands: 1994. pp. 87–95. [Google Scholar]
- Morris V.B. Apluteal development of the sea urchin Holopneustes purpurescens Agassiz (Echinodermata: Echinoidea: Euechinoidea) Zool. J. Linn. Soc. 1995;114:349–364. doi:10.1006/zjls.1995.0028 [Google Scholar]
- Morris V.B, Byrne M. Involvement of two Hox genes and Otx in echinoderm body-plan morphogenesis in the sea urchin Holopneustes purpurescens. J. Exp. Zool. B Mol. Dev. Evol. 2005;304B:456–467. doi: 10.1002/jez.b.21065. doi:10.1002/jez.b.21065 [DOI] [PubMed] [Google Scholar]
- Ohshima H. On the development of Cucumaria echinata v. Marenzeller. Q. J. Microsc. Sci. 1921;65:173–246. [Google Scholar]
- Paul C.R.C, Smith A.B. The early radiation and phylogeny of echinoderms. Biol. Rev. 1984;59:443–481. doi:10.1086/414043 [Google Scholar]
- Peterson K.J, Arenas-Mena C, Davidson E.H. The A/P axis in echinoderm ontogeny and evolution: evidence from fossils and molecules. Evol. Dev. 2000;2:93–101. doi: 10.1046/j.1525-142x.2000.00042.x. doi:10.1046/j.1525-142x.2000.00042.x [DOI] [PubMed] [Google Scholar]
- Runnström Sv. Über die Entwicklung von Leptosynapta inhaerens. Bergens Mus. Årbok. 1927;1:1–80. [Google Scholar]
- Saucède T, Mooi R, David B. Combining embryology and paleontology: origins of the anterior–posterior axis in echinoids. C. R. Palevol. 2003;2:399–412. doi:10.1016/j.crpv.2003.09.017 [Google Scholar]
- Saucède T, Mooi R, David B. Evolution to the extreme: origins of the highly modified apical system in pourtalesiid echinoids. Zool. J. Linn. Soc. 2004;140:137–155. doi:10.1111/j.1096-3642.2004.t01-1-00091.x [Google Scholar]
- Smiley S. Metamorphosis of Stichopus californicus (Echinodermata: Holothuroidea) and its phylogenetic implications. Biol. Bull. 1986;171:611–631. doi: 10.2307/1541627. doi:10.2307/1541627 [DOI] [PubMed] [Google Scholar]
- Smith A.B, Peterson K.J, Wray G, Littlewood D.T.J. From bilateral symmetry to pentaradiality. In: Cracraft J, Donoghue M.J, editors. Assembling the tree of life. Oxford University Press; New York, NY: 2004. pp. 365–383. [Google Scholar]
- Swalla B.J. Building divergent body plans with similar genetic pathways. Heredity. 2006;97:235–243. doi: 10.1038/sj.hdy.6800872. doi:10.1038/sj.hdy.6800872 [DOI] [PubMed] [Google Scholar]
- von Ubisch L. Die Entwicklung von Strongylocentrotus lividus. (Echinus microtuberculatus, Arbacia pustulosa.) Zeit. f. wiss. Zool. 1913;106:409–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in the labelling of the 2+1+1 and the 1+3+1 ambulacral patterns are explained