Abstract
Although no naturally infected sheep with bovine spongiform encephalopathy (BSE) has ever been discovered, it remains possible that BSE once infected the UK sheep population, has been transmitted between sheep, and is still present today. We constructed a mathematical model to assess the current maximum theoretical exposure to consumers from BSE-infected ovine material and to estimate the risk reduction that could be achieved by abattoir-based control options if BSE-infected sheep were ever found in the national flock. We predict that, if present, the exposure to consumers from a single BSE-infected sheep would be high: one sheep, close to the end of its incubation period, is likely to contribute 10–1000 times more infectious material than a fully infectious cow. Furthermore, 30% of this exposure comes from infectivity residing in lymphatic and peripheral tissue that cannot be completely removed from a carcass.
We are 95% confident that throughout Great Britain, no more than four sheep flocks currently harbour an ongoing BSE epidemic. However, since the exposure from a single infected sheep is high, the annual human exposure from four ‘typical’ BSE-infected flocks could be considerable. Small reductions in exposure could be achieved by strategies based on tissue testing, a 12-month age restriction or expanded definitions of high-risk tissues. A six-month age restriction is likely to be more effective and genotype-based strategies the most effective.
Keywords: ovine, bovine spongiform encephalopathy, mathematical model, epidemiology, risk analysis
1. Introduction
Since a link was made between bovine spongiform encephalopathy (BSE) and variant Creutzfeldt–Jakob disease (vCJD; Collinge et al. 1996; Bruce et al. 1997; Hill et al. 1997; Scott et al. 1999), there has been concern that BSE may have infected sheep which were fed the same contaminated feed supplements that caused the BSE epidemic in cattle. Speculation has been driven by experimental results showing that sheep are susceptible to BSE infection from intravenous, intracerebral (ic) and oral challenge (Foster et al. 2001a,b; Jeffrey et al. 2001; Houston et al. 2003). Furthermore, a natural case of BSE infection in a French goat has recently been confirmed (Froissart 2004), and preliminary results suggest that a second infected goat has been located in Scotland (DEFRA 2005). However, the Veterinary Laboratories Agency (VLA) has tested 2368 ovine TSE cases from 450 flocks reporting scrapie between 2001 and 2004, and none has shown a pattern clearly indicating a case of BSE (Stack et al. 2006). This is promising, but it must be noted that a recent study estimated that only 38% of farmers notice scrapie actually report it (Sivam et al. 2003, 2006).
Even if BSE did infect the sheep population, it is questionable whether further transmission from sheep to sheep would have occurred. Neither goat case provides any insight into the likelihood of ongoing transmission because both animals were alive before comprehensive feed bans were in place in their respective countries. However, it has recently been shown that natural transmission of BSE can occur between an experimentally infected mother and her lamb (Bellworthy et al. 2005a). Scrapie does transmit between sheep and the similarities in pathogenesis of these two diseases imply that horizontal transmission of ovine BSE is a real possibility. If further transmission has occurred naturally, it is most probable that the epidemic quickly died out or is still on the decline, but there is a remote possibility that we are at the start of an ovine BSE epidemic that will continue to grow (Ferguson et al. 2002; Kao et al. 2002).
Ovine susceptibility to TSEs is largely controlled by polymorphism at the gene encoding the prion protein (PrP; Clouscard et al. 1995; Hunter et al. 1996). The principal mutations associated with susceptibility to disease are located at codons 136 (alanine (A) or valine (V)), 154 (arginine (R) or histidine (H)) and 171 (glutamine (Q), R or H). The genotype most susceptible to BSE (ARQ/ARQ) is common in British flocks.
Why should we care about an outbreak of ovine BSE? One of the main concerns centres on the distribution of infectivity through sheep tissues. While infectivity in cattle is largely confined to the nervous system (Wells et al. 1998), the same is not true for sheep. BSE infectivity and disease-associated prion protein have been found to be widespread throughout the body of an infected sheep (Foster et al. 2001b; Jeffrey et al. 2001; Ferguson et al. 2002; Bellworthy et al. 2005b; Gonzalez et al. 2005), even in the early stages of infection. Furthermore, a number of tissues that have been shown to carry infectivity are currently eaten by humans and cannot be completely removed from a sheep carcass.
The aim of this study is to quantify the exposure to consumers to BSE-infected sheep meat. We estimate the maximum number of flocks in Great Britain that could currently be harbouring BSE and the total infectious burden that these flocks could contribute to the human food chain. We also quantify the probable impact of a range of control options. This study collates data from a wide range of sources, including the 2002 anonymous scrapie postal survey (Sivam et al. 2003, 2006) and the Institute for Animal Health (IAH) farm-based scrapie flock survey (Baylis et al. 2000), and published data on studies of natural scrapie (Hadlow et al. 1982; Andreoletti et al. 2000; van Keulen et al. 2000, 2002) and experimental BSE (Foster et al. 2001b; Jeffrey et al. 2001; Ferguson et al. 2002; Houston et al. 2003; Bellworthy et al. 2005b; Gonzalez et al. 2005). These data are used to parametrize a mathematical model that tracks rising infectivity within infected sheep, sheep-to-sheep transmission within a flock and the rate at which sheep leave flocks to enter the food chain.
2. Material and methods
The process by which BSE-infected sheep meat could enter the food chain can be considered at three levels: the accumulation of BSE infectivity within a single sheep (figure 1); the spread of BSE infection among sheep in a single flock (figure 2); and the number of BSE-infected flocks in Britain (figure 3). We describe a mathematical model that gives a dynamic description of the infectious burden in sheep in hypothetical infected flocks. This model generates predictions about the flow of this infectious burden from infected flocks into the human food chain and allows us to compare the impact of different control options.
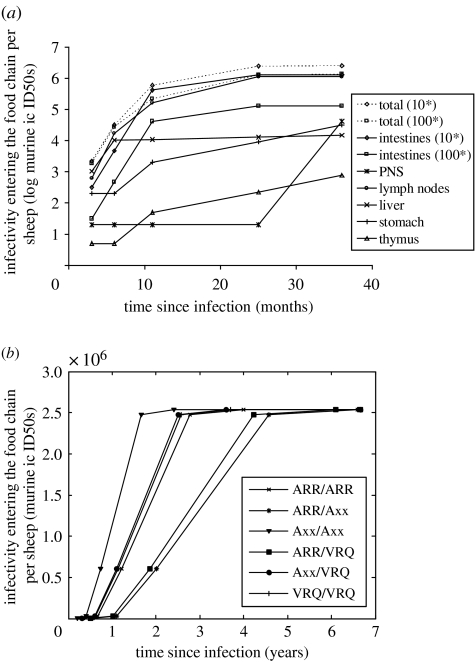
Figure 1.
(a) Estimated infectious dose entering the food chain from one sheep over the course of an infection. (b) Inferred infectiousness of sheep by genotype. Infectivity entering the food chain per sheep is estimated by considering tissue weight, the proportion of each tissue that enters the food chain and infectivity in each tissue. Tissue infectivity is estimated from results of mouse bioassay experiments and tests for disease-specific PrP in sheep with natural scrapie and experimental BSE (Hadlow et al. 1982; Andreoletti et al. 2000; van Keulen et al. 2000, 2002; Foster et al. 2001a; Bellworthy et al. 2005b). Asterisk, the processing of the manure-stripped duodenum and jejunum into sausage casings is assumed to result in a reduction in infectivity in these tissues before consumption. The magnitude of that reduction is controversial. Two scenarios are considered: a further reduction in infectivity by a factor of 10 and a further reduction by a factor of 100. In figure (b), the conservative factor 10 reduction is used.
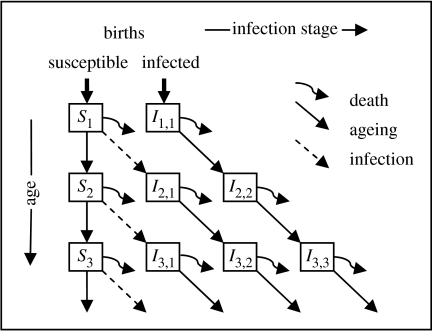
Figure 2.
A within-flock model of TSE infection and disease progression. Si represents the number of susceptible sheep in the flock aged i. Ii,j represents the number of sheep in the flock that are aged i and have been infected for time j. These two state variables are further subdivided by sex and genotype as shown in table 1.
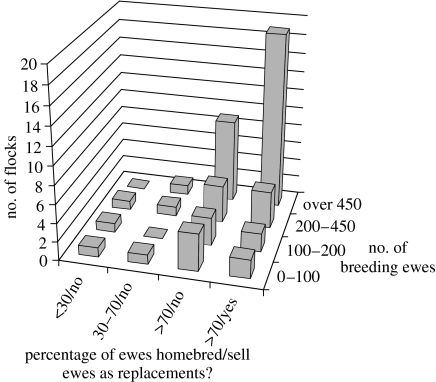
Figure 3.
Size and breeding status of flocks with a scrapie epidemic. Data from the 2002 anonymous scrapie postal survey, conducted by the VLA.
(a) BSE infectivity in a single sheep over the course of an infection
To estimate the infectivity in a single sheep over the course of a BSE infection, we collated published data on the distribution of infectivity and abnormal PrP in natural scrapie cases and experimental BSE cases. Genotype is a strong determinant of the progression of clinical disease within an animal, but no genotype-specific tissue data are available; so, in the models used here, infectivity accumulates as a function of the average incubation period. These estimates of tissue infectivity were combined with estimates of the tissue weight and the proportion of each tissue entering the human food chain to calculate the total exposure to humans from a single infected sheep.
(b) Within-flock spread of infectivity
We have created a difference equation model that simultaneously encapsulates both within-sheep and within-flock dynamics of sheep TSEs. The model has full age structure and a full description of the inheritance and impact of the PrP gene.
We have developed a new classification for TSE alleles that combines ARQ, ARH and AHQ into one group of alleles which we call Axx. This simplification of PrP genetics is similar to the NSP classification, except that it has a separate group for genotype VRQ/VRQ. It allows us to model the genetics of TSE susceptibility as a single-locus, three-allele system, where the three alleles are ARR, Axx and VRQ. Properties of the six resulting genotypes are described in table 1 and figure 1.
Table 1.
Sheep genotypes: prevalence, susceptibility and incubation period.
| genotype | prevalence of genotypea (%) | susceptibility to intracerebral BSE challenge | mean incubation period after BSE intracerebral challengeb (years) | ||
|---|---|---|---|---|---|
| national scrapie plan screening | abattoir screening | number positive/number challengedb | relative | ||
| ARR/ARR | 26.3 | 19.5 | 3/19 | 0.1 | 3.0 |
| ARR/Axx | 40.8 | 41.9 | 3/24 | 0.14 | 5.6 |
| Axx/Axx | 25.5 | 26.5 | 19/21 | 1 | 1.4 |
| ARR/VRQ | 3.4 | 5.5 | 1/11 | 0.14 | 5.1 |
| Axx/VRQ | 3.7 | 6.2 | 3/11 | 0.30 | 2.6 |
| VRQ/VRQ | 0.2 | 0.4 | 4/10 | 0.44 | 2.7 |
Jeffrey et al. (2001), Houston et al. (2003) and Bellworthy et al. (2005b). The allele labelled Axx represents alleles ARQ, AHQ and ARH.
The model has three independent variables: time, t; age, i; and time since infection, j. There are two state variables: Xg,i,s(t), the number of susceptible sheep of genotype g, age i and sex s at time t; and Yg,i,j,s(t), the number of infected sheep of genotype g, age i, infected for time j and sex s at time t. The model consists of a description of disease transmission and progression that is superimposed on a model of Mendelian inheritance of the PrP gene and the demography of a flock. Data on the age (McLean et al. 1999) and genotype distribution of sheep in surveyed flocks were used to parametrize the genetics and demography. Sheep leave the flock to be slaughtered at an age and time-specific rate that was obtained from published slaughter statistics (DEFRA 2002). The auxiliary variable describes infected sheep that enter the human food chain of genotype g, age i, infected for time j and sex s at time t. Variables, parameters and equations describing the model in more detail are presented in §§ 4 and 5 of the electronic supplementary material.
The model allows for both horizontal and vertical (including perinatal) transmission of BSE. Data on attack rates after experimental challenge of sheep with BSE were used to set the relative susceptibility of different genotypes (table 1) and the absolute transmission rate was set to give a within-flock epidemic that would yield 10 clinical cases of BSE in a flock of 1067 sheep in the ninth year of an epidemic. This size of epidemic is consistent with observations of scrapie-affected flocks (see figure 4 of electronic supplementary material).
Published results on the incubation period after intracerebral BSE challenge were used to infer an incubation period for each genotype (Jeffrey et al. 2001; Houston et al. 2003; Bellworthy et al. 2005b). By analogy with scrapie (see table 4 of electronic supplementary material), we assume that natural BSE incubation periods are equal to the mean incubation period after intracerebral challenge plus 1 year (table 1). The distributions of incubation periods for each genotype follow a gamma distribution with shape parameter 7.3, which is equal to that calculated for the distribution of the age of reported scrapie cases (data from the VLA). This is consistent with the model's assumption that most sheep get infected in the first few months of life. The rate of accumulation of infectivity within an infected sheep is inferred from the infectivity data in figure 1a and the incubation periods in table 1.
The model's equations and parameters and detailed descriptions of parameter estimates are given in the electronic supplementary material. The model generates a dynamic description of the total burden of infectivity generated from one hypothetical flock into the human food chain as a BSE epidemic progresses.
(c) The number of BSE flocks
The clinical signs of scrapie and ovine BSE are indistinguishable, and it has been suggested that BSE has never been discovered in sheep because the endemic scrapie problem is masking BSE. The VLA tests reported sheep TSE cases for BSE. Samples from 2368 cases from 450 flocks (dating from 1 January 1998 to 31 May 2004) have been tested and none has shown a pattern clearly indicating BSE. This translates to a best estimate that no flocks are infected with BSE, but also provides an upper 95% confidence limit for the proportion of flocks with TSE cases that could be BSE as 0.66% (Stack et al. 2006).
It is important to distinguish flocks with an ongoing TSE epidemic (either BSE or scrapie) from those that have bought a TSE-infected animal that did not go on to ignite a within-flock epidemic. To estimate the number of flocks that could be harbouring an ongoing BSE epidemic, we analysed data from the 2002 scrapie postal survey. Such a flock is defined here as a holding that has had at least one case of homebred scrapie over the last year or at least two cases of scrapie in the last 5 years, of which at least one was homebred. We estimate that, at present, there are approximately 580 flocks with an ongoing TSE epidemic (figure 3). In addition, there are another 1000 flocks that have seen scrapie over the last 5 years, but only in bought-in animals.
By combining these two calculations, we conclude that the upper 95% confidence interval (CI) for the maximum number of flocks harbouring a BSE epidemic is four. In what follows, we explore the impact of various risk reduction strategies if they were applied to four different BSE-infected flock models. The size and underlying demography of each of the four flocks are chosen to mimic four typical scrapie-affected flocks and are described in table 2 of the electronic supplementary material.
3. Results
(a) The exposure from a single sheep
Collated data from experimental BSE infections and natural scrapie infections imply that the infectious burden in sheep infected with either TSE rises rapidly after infection and is high in peripheral tissues (see figure 1a and the electronic supplementary material, §3.3). Data from experimental BSE infections are largely qualitative and for quantitative measures, Hadlow's data on natural scrapie infections (Hadlow et al. 1982) remain the best available source of information. Although extrapolation from scrapie to BSE introduces inaccuracies, it is clear that BSE-infected sheep carry a large burden of infectivity in a wide array of tissues. Many of the tissues that carry infectivity currently enter the human food chain (see table 10 of electronic supplementary material) and some of the most heavily infected tissues are located within the muscle tissue that could not conceivably be wholly removed from a carcass. Recent studies indicate that there is also pathogenic prion protein (PrPSc) in blood (Houston et al. 2000; Hunter et al. 2002; Siso et al. 2006) and muscle tissue (Andreoletti et al. 2004).
Current legislation prevents certain ovine specified risk material (SRM) from entering the food chain. The tissues currently banned are the brain, eyes, spinal cord and tonsils of sheep over 12 months, and the spleen and ileum of all sheep. Some of the remaining tissues that carry infectivity are not used in human food. However, a number of tissues that would carry infectivity do enter the human food chain—the lymph nodes, liver, pancreas, peripheral nervous system (PNS), the duodenum and jejunum of the small intestine, and the rumen and reticulum of the stomach. It is the potential for such tissues to carry ovine BSE into the human food chain that we estimate here. This report does not consider the exposure from blood or muscle tissue as the infectivity of such tissues has never been quantified.
We have estimated that one infected sheep, two-thirds of its way through the incubation period, would contribute around 2.3 million murine ic ID50s to the food chain under current SRM legislation. The majority of this can be attributed to the exposure from lymph nodes (1.1 million) and intestines (1.2 million). At six months post-infection, the exposure from a single sheep of genotype Axx/Axx is 0.23 million murine ic ID50s. At this stage, the intestines (129 000 ic ID50s), lymph nodes (62 000 ic ID50s) and the liver (11 000 ic ID50s) are the most infectious.
How does this compare to the human exposure from cattle? One report (Comer & Huntly 2004) states that, at present, the exposure from one fully infectious cow would be approximately 27 bovine oral ID50s. Titration experiments, challenging mice (intracerebrally) and cattle (orally) with CNS tissue from naturally infected cattle provide some guide to the conversion rate between bovine oral ID50s and murine ic ID50s. Murine ic titres have been shown to vary between 103 and 105 ID50s g−1 CNS tissue and bovine oral titres are estimated to be 10 ID50s g−1 CNS tissue (SSC 2000). These figures suggest that to convert from bovine oral ID50s to murine ic ID50s requires an increase by 2–4 orders of magnitude. Even if we assume the most conservative estimate, an increase by four orders of magnitude, the infectivity from one cow corresponds to 0.27 million murine ic ID50s. This is 10 times less than the exposure from the sheep. To further put these figures into perspective, the total exposure from cattle in 2006 is estimated at around 50 bovine oral ID50s (Ferguson & Donnelly 2003; 0.005–0.5 million murine ic ID50s). Comparisons of this sort must be viewed in light of the uncertainties that remain in estimating not only the conversion rate between bovine and murine titres, but also the absolute exposure from cattle.
(b) Exposure from four BSE-infected flocks
Based upon results from testing reported scrapie cases for BSE, and from data provided in the scrapie postal survey, we report that the upper 95% CI estimate for the number of flocks in Great Britain with a BSE epidemic is four. Using our model, four ‘typical’ TSE-infected flocks would contribute around 20–30 million murine ic ID50s to the food chain each year (table 2). The vast majority of this comes from the single largest flock (see table 2 of electronic supplementary material). The estimated total exposure from cattle since 1980, of 54 million bovine oral ID50s (Comer & Huntly 2004; 5400–540 000 million murine ic ID50s), indicates that the yearly exposure from BSE-infected sheep flocks is 0.005–0.5% of the total exposure from cattle since 1980 if it is present at all.
Table 2.
The effect of risk reduction strategies on the infectious dose entering the food chain from four example flocks harbouring a BSE epidemic.
| risk reduction strategy | infectious dose entering food chain per year (millions of murine ic ID50s) | ||
|---|---|---|---|
| ARR/ARR resistant | ARR/ARR susceptiblea | ||
| current legislation | 23.9 | 27.1 | |
| 1 | test all for PrPSc | 9.01 | 9.83 |
| 2 | test all over 12 months for PrPSc | 10.1 | 11.0 |
| 3 | current SRM extended to all ages plus intestines, stomach, liver, thymus and 30% lymph nodes | 6.66 | 7.58 |
| 4 | only eat lamb under 12 months | 9.07 | 9.84 |
| 5 | only eat lamb under 6 months | 1.64 | 1.76 |
| 6 | eat ARR/VRQ and ARR/Axx under 12 months and all ARR/ARR | 0.05 | 1.80 |
| 7 | (6) plus eat 1% of all other sheep under 12 months | 0.14 | 1.90 |
| 8 | (6) plus eat 5% of all other sheep under 12 months | 0.50 | 2.28 |
| 9 | eat ARR/ARR under 18 months, and ARR/Axx and ARR/VRQ under 6 months | 0.01 | 0.10 |
| 10 | (3) plus only eat lamb under 12 months | 1.99 | 2.17 |
| 11 | (3) plus only eat lamb under 6 months | 0.40 | 0. 43 |
| 12 | (6) plus (3) | 0.01 | 0.57 |
| 13 | (9) plus (3) | 0.003 | 0.03 |
ARR/ARR sheep have a relative susceptibility of 0.1 and mean incubation period of 4 years.
(c) Impact of risk reduction strategies on four infected flocks
If BSE were discovered in the national sheep flock, what more could be done to protect consumers? In what follows, we consider the impact of various strategies that could be implemented at the abattoir level: PrPSc tests, age restrictions, tighter tissue-based controls and genotype-based exclusion (table 2). Of these options, PrPSc testing, tightened SRM legislation and a 12-month age restriction perform the worst. A six-month age restriction has intermediate impact, and genotyping strategies that involve exclusion of all but the most resistant genotypes (those carrying an ARR allele) perform the best. Even under these controls, the exposure from four sheep flocks is likely to be equivalent to the current exposure from the UK cattle population.
We have also estimated the risk reduction achieved under combinations of these strategies, the most effective of which would be a tight genotype and age-based restriction combined with stricter SRM legislation.
(i) PrPSc testing (strategies 1 and 2)
Strategies 1 and 2 involve testing sheep brains for PrPSc. Inevitably, the impact of such a strategy is directly linked to the sensitivity of the test used. Published data on testing infected sheep brains for PrPSc indicate that such tests perform poorly in the early stages of infection; the earliest that PrPSc has been detected in a TSE-infected brain is at 9–10 months post-infection (Andreoletti et al. 2000; van Keulen et al. 2002), while in the same experiments, PrPSc was detected in lymph nodes at only 2–3 months post-infection. Based on published data (Jeffrey et al. 2001; van Keulen et al. 2002; Bellworthy et al. 2005b), we make conservative estimates of test sensitivity (see table 7 of electronic supplementary material) that are reflected in poor performance from these strategies, reducing the infectious load entering the food chain from four flocks by around 60%.
(ii) Tighter SRM (strategy 3)
We have considered the impact of tightening the SRM legislation to span a wider range of tissues that pose a potential risk to consumers at present. In practice, not all infectious tissues could be removed from a carcass. The PNS and the lymphoreticular system extend throughout the carcass and full removal would be costly, if not impossible.
Here, we model the risk reduction that is achieved by adding the liver, pancreas, stomach, intestines and 30% of the lymph nodes to the list of SRM. Under this assumption, the exposure from a single sheep, two-thirds of its way through an infection, would drop from 2.3 to 0.8 million murine ic ID50s. Most of this reduction is attributable to the further removal of the intestines and lymph nodes. The exposure from a six-month infected Axx/Axx sheep would drop from 0.20 to 0.04 million murine ic ID50s. At this age, removal of the liver is also significant.
The exposure from four flocks, under this tightened SRM strategy, is reduced by 72%. The residual 28% results from the difficulty in removing the PNS and all the lymph nodes, both of which have a high level of infectivity, particularly later on in the incubation period.
(iii) Age restrictions (strategies 4 and 5)
We modelled the impact of age restrictions that prevent sheep over 12 or 6 months from entering the human food chain. These strategies would reduce the infectious load from four flocks by 63 and 93%, respectively. These strategies perform poorly because Axx homozygous sheep (27% of the UK flock) are easily infected and have a short incubation period. They are therefore likely to bear a heavy burden of infectivity by six months of age. At 12 months of age, these sheep are likely to be nearly halfway through their incubation period and harbouring 1.1 million murine ic ID50s.
(iv) Genotyping combined with age restrictions
Sheep genotype confers susceptibility to TSE infection and affects the time from infection to onset of clinical disease. Sheep of different genotypes thus pose different levels of risk to consumers. Possible control options could restrict the genotypes that may enter the food chain.
Eat only ARR/ARR (all ages) and ARR/—under 12 months (strategies 6–8). The ARR allele has long been associated with resistance to ovine TSEs. No ARR homozygous sheep has yet been discovered with clinical scrapie, though a number of atypical scrapie infections have been found in sheep with this genotype (Everest et al. 2006), and experiments suggest they are also the least susceptible to BSE. We have modelled the impact of a control option that involves allowing only ARR homozygous sheep (all ages) and ARR heterozygous sheep that are under 12 months old into the food chain. In the event that ARR homozygotes are completely resistant, there is a 99.8% risk reduction, making it one of the more effective strategies considered here. It is, however, still not perfect because ARR heterozygotes are susceptible and could pose a risk, even before 12 months of age.
It is debatable whether or not ARR homozygous sheep would, in fact, be resistant to BSE under natural circumstances as oral and intracerebral challenges have proved successful (Houston et al. 2003; Gonzalez et al. 2005; Andreoletti et al. 2006). Clearly, this control option is less effective if ARR homozygotes are susceptible to natural infection. This is reflected in a less promising estimate that the exposure is reduced by only 93% under the assumption that these sheep are marginally more resistant than ARR/Axx sheep and have an average incubation period of 4 years.
If genotype testing were imperfect (so that a percentage of sheep of other genotypes were mistakenly allowed into the human food chain), then these strategies become significantly less effective (strategies 7 and 8).
Eat ARR/ARR under 18 months and ARR/— under six months (strategy 9). A tighter genotyping strategy could also include an age restriction of, say, 18 months on ARR homozygotes and six months on ARR heterozygotes. This would be more effective, reducing the exposure by 99.9 and 99.6%, to 0.01 and 0.10 million murine ic ID50s, if homozygous sheep are resistant and susceptible, respectively. These figures lie within the estimated range for the total exposure from cattle in 2006, 0.005–0.5 million murine ic ID50s.
(v) Combination strategies (strategies 10–13)
Not surprisingly, combination strategies are more effective than single strategies. We considered four strategies that combined maximum SRM removal with age and genotype restrictions. The tightest of these (strategy 13) allows only ARR homozygotes under 18 months and ARR heterozygotes under six months into the food chain and further imposes a maximum realistic SRM removal scheme. If ARR homozygotes are completely resistant to BSE infection, then this strategy reduces infectivity in the human food chain by four orders of magnitude. The strategy has 10-fold less impact if ARR homozygotes are somewhat susceptible.
4. Discussion
We estimate that there are at most only four flocks currently harbouring a BSE epidemic in Britain, but that even a single BSE-infected sheep could pose a considerable risk to consumers, contributing 10–1000 times as much infectivity in the human food chain as a fully infectious cow. Furthermore, 30% of the exposure from sheep comes from infectivity residing in lymph nodes and the PNS—tissues that cannot feasibly be entirely removed from a carcass.
The exposure from four ‘typical’ BSE-infected sheep flocks each year could be considerable. Our models predict that only a small reduction in exposure could be achieved by a PrPSc-testing based strategy, a 12-month age restriction or a tightened tissue-based strategy. A six-month age restriction is likely to be more effective and genotype-based strategies, which allow only the most resistant genotypes to enter the food chain, will achieve the greatest reduction in risk to consumers.
All the options discussed here are currently under consideration by the authorities in contingency planning for the event that ovine BSE is discovered. Such decisions, however, must also take into account the predicted cost and feasibility of different plans. Genotyping all sheep in the UK would be extremely expensive, if at all possible or accurate, on such a large scale. Furthermore, it would remove a high proportion of sheep from the food chain, which would also be the disadvantage of having a strict age-based cut-off. Testing sheep for PrPSc would be less expensive overall as it would not waste vast numbers of uninfected sheep. Tighter SRM-based strategies are also likely to be relatively cheap since only an extension of the existing SRM procedure would be required.
Our calculations rest upon a straightforward mathematical model, but are necessarily data hungry in a situation where not all the relevant data have yet been gathered. The modelling exercise usefully highlights the most glaring gaps in our knowledge. For calculating the infectious load produced by a BSE-infected sheep flock, the most important new data would be quantified BSE infectivity in different tissues in sheep of different genotypes at 6 and 12 months of age (after infection very close to birth). For comparisons of the impact of different risk reduction strategies, information on the sensitivity of proposed tests by genotype, tissue and time since infection would be particularly useful. The comparative risk of ovine and bovine BSE requires further analysis of the conversion rate from bovine oral ID50s to murine ic ID50s. The range used here of 2–4 orders of magnitude probably does not reflect all of the uncertainty surrounding this estimate. Furthermore, comparisons between the exposure from bovine and ovine BSE must be viewed in the light of the uncertainties surrounding estimates of the exposure from cattle.
Although gaps exist in our detailed knowledge of the dynamics of BSE infectious load in infected sheep, our conclusions are robust to the uncertainties that remain and provide best estimates of the exposure from ovine BSE and the effectiveness of control options which can be used in contingency planning. This is the only study that assesses the impact of genotype-based strategies and compares them with other options.
Our main conclusion is that we should remain vigilant of ovine BSE, simply because even a single recently infected sheep is likely to harbour considerable infectivity throughout the carcass, including in tissues that could not feasibly be removed at the abattoir. Furthermore, despite much positive news in recent years, a slowly developing ovine BSE epidemic is not inconceivable and the genotype of sheep that would most easily be infected and in which disease progresses most quickly is very common in our sheep flocks.
Acknowledgments
H.F. was funded by the Food Standards Agency, grant no. M03027.
We would also like to thank Marie McIntyre for her help in collecting data.
Supplementary Material
The model superimposes the spread of BSE upon an age-structured demographic model of sheep in a flock which includes Medelian inheritance of PrP genes. A detailed summary of TSE data used to parameterise the model is also presented
References
- Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen J.M, Lantier F. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 2000;81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- Andreoletti O, et al. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat. Med. 2004;10:591–593. doi: 10.1038/nm1055. doi:10.1038/nm1055 [DOI] [PubMed] [Google Scholar]
- Andreoletti O, et al. Bovine spongiform encephalopathy agent in spleen from an ARR/ARR orally exposed sheep. J. Gen. Virol. 2006;87:1043–1046. doi: 10.1099/vir.0.81318-0. doi:10.1099/vir.0.81318-0 [DOI] [PubMed] [Google Scholar]
- Baylis M, Houston F, Goldmann W, Hunter N, McLean A.R. The signature of scrapie: differences in the PrP genotype profile of scrapie-affected and scrapie-free UK sheep flocks. Proc. R. Soc. B. 2000;267:2029–2035. doi: 10.1098/rspb.2000.1245. doi:10.1098/rspb.2000.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellworthy S.J, et al. Natural transmission of BSE between sheep within an experimental flock. Vet. Rec. 2005a;157:206. doi: 10.1136/vr.157.7.206. [DOI] [PubMed] [Google Scholar]
- Bellworthy S.J, et al. Tissue distribution of bovine spongiform encephalopathy infectivity in Romney sheep up to the onset of clinical disease after oral challenge. Vet. Rec. 2005b;156:197–202. doi: 10.1136/vr.156.7.197. [DOI] [PubMed] [Google Scholar]
- Bruce M.E, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. doi:10.1038/39057 [DOI] [PubMed] [Google Scholar]
- Clouscard C, et al. Different allelic effects of the codons 136 and 171 of the prion protein gene in sheep with natural scrapie. J. Gen. Virol. 1995;76(Pt 8):2097–2101. doi: 10.1099/0022-1317-76-8-2097. [DOI] [PubMed] [Google Scholar]
- Collinge J, Sidle K.C, Meads J, Ironside J, Hill A.F. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. doi:10.1038/383685a0 [DOI] [PubMed] [Google Scholar]
- Comer P.J, Huntly P.J. Exposure of the human population to BSE infectivity over the course of the BSE epidemic in the United Kingdom and the impact of changes to the Over Thirty Month Rule. J. Risk Res. 2004;7:523–543. doi:10.1080/1366987032000123865 [Google Scholar]
- DEFRA. 2002 Slaughter statistics 2002. See http://statistics.defra.gov.uk/esg/datasets/slaughw.xls
- DEFRA. 2005 Possible BSE in a 1990 UK goat sample.
- Everest S.J, Thorne L, Barnicle D.A, Edwards J.C, Elliott H, Jackman R, Hope J. Atypical prion protein in sheep brain collected during the British scrapie-surveillance programme. J. Gen. Virol. 2006;87:471–477. doi: 10.1099/vir.0.81539-0. doi:10.1099/vir.0.81539-0 [DOI] [PubMed] [Google Scholar]
- Ferguson N.M, Donnelly C.A. Assessment of the risk posed by bovine spongiform encephalopathy in cattle in Great Britain and the impact of potential changes to current control measures. Proc. R. Soc. B. 2003;270:1579–1584. doi: 10.1098/rspb.2003.2484. doi:10.1098/rspb.2003.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M, Ghani A.C, Donnelly C.A, Hagenaars T.J, Anderson R.M. Estimating the human health risk from possible BSE infection of the British sheep flock. Nature. 2002;415:420–424. doi: 10.1038/nature709. doi:10.1038/nature709 [DOI] [PubMed] [Google Scholar]
- Foster J.D, Parnham D, Chong A, Goldmann W, Hunter N. Clinical signs, histopathology and genetics of experimental transmission of BSE and natural scrapie to sheep and goats. Vet. Rec. 2001a;148:165–171. doi: 10.1136/vr.148.6.165. [DOI] [PubMed] [Google Scholar]
- Foster J.D, Parnham D.W, Hunter N, Bruce M. Distribution of the prion protein in sheep terminally affected with BSE following experimental oral transmission. J. Gen. Virol. 2001b;82:2319–2326. doi: 10.1099/0022-1317-82-10-2319. [DOI] [PubMed] [Google Scholar]
- Froissart, R. 2004 Progress report on actions from the meeting on 25th November, 2004: the TSE community reference laboratory expert group on strains.
- Gonzalez L, Martin S, Houston F.E, Hunter N, Reid H.W, Bellworthy S.J, Jeffrey M. Phenotype of disease-associated PrP accumulation in the brain of bovine spongiform encephalopathy experimentally infected sheep. J. Gen. Virol. 2005;86:827–838. doi: 10.1099/vir.0.80299-0. doi:10.1099/vir.0.80299-0 [DOI] [PubMed] [Google Scholar]
- Hadlow W.J, Kennedy R.C, Race R.E. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- Hill A.F, Desbruslais M, Joiner S, Sidle K.C, Gowland I, Collinge J, Doey L.J, Lantos P. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. doi:10.1038/38925 [DOI] [PubMed] [Google Scholar]
- Houston F, Foster J.D, Chong A, Hunter N, Bostock C.J. Transmission of BSE by blood transfusion in sheep. Lancet. 2000;356:999–1000. doi: 10.1016/s0140-6736(00)02719-7. doi:10.1016/S0140-6736(00)02719-7 [DOI] [PubMed] [Google Scholar]
- Houston F, Goldmann W, Chong A, Jeffrey M, Gonzalez L, Foster J, Parnham D, Hunter N. Prion diseases: BSE in sheep bred for resistance to infection. Nature. 2003;423:498. doi: 10.1038/423498a. doi:10.1038/423498a [DOI] [PubMed] [Google Scholar]
- Hunter N, Foster J.D, Goldmann W, Stear M.J, Hope J, Bostock C. Natural scrapie in a closed flock of Cheviot sheep occurs only in specific PrP genotypes. Arch. Virol. 1996;141:809–824. doi: 10.1007/BF01718157. doi:10.1007/BF01718157 [DOI] [PubMed] [Google Scholar]
- Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. Transmission of prion diseases by blood transfusion. J. Gen. Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- Jeffrey M, Ryder S, Martin S, Hawkins S.A, Terry L, Berthelin-Baker C, Bellworthy S.J. Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. J. Comp. Pathol. 2001;124:280–289. doi: 10.1053/jcpa.2001.0465. doi:10.1053/jcpa.2001.0465 [DOI] [PubMed] [Google Scholar]
- Kao R.R, Gravenor M.B, Baylis M, Bostock C.J, Chihota C.M, Evans J.C, Goldmann W, Smith A.J, McLean A.R. The potential size and duration of an epidemic of bovine spongiform encephalopathy in British sheep. Science. 2002;295:332–335. doi: 10.1126/science.1067475. doi:10.1126/science.1067475 [DOI] [PubMed] [Google Scholar]
- McLean A.R, Hoek A, Hoinville L.J, Gravenor M.B. Scrapie transmission in Britain: a recipe for a mathematical model. Proc. R. Soc. B. 1999;266:2531–2538. doi: 10.1098/rspb.1999.0956. doi:10.1098/rspb.1999.0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.R, Will R, Ironside J, Nguyen H.O, Tremblay P, DeArmond S.J, Prusiner S.B. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc. Natl Acad. Sci. USA. 1999;96:15 137–15 142. doi: 10.1073/pnas.96.26.15137. doi:10.1073/pnas.96.26.15137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siso S, Gonzalez L, Houston F, Hunter N, Martin S, Jeffrey M. The neuropathological phenotype of experimental ovine BSE is maintained after blood transfusion. Blood. 2006;108:745–748. doi: 10.1182/blood-2005-12-5156. doi:10.1182/blood-2005-12-5156 [DOI] [PubMed] [Google Scholar]
- Sivam S.K, Baylis M, Gravenor M.B, Gubbins S, Wilesmith J.W. Results of a postal survey in 2002 into the occurrence of scrapie in Great Britain. Vet. Rec. 2003;153:782–783. [PubMed] [Google Scholar]
- Sivam S.K, Baylis M, Gravenor M.B, Gubbins S. Descriptive analysis of the results of an anonymous postal survey of the occurrence of scrapie in Great Britain in 2002. Vet. Rec. 2006;158:501–506. doi: 10.1136/vr.158.15.501. [DOI] [PubMed] [Google Scholar]
- SSC. 2000 EC, Scientific Steering Committee. Opinion: oral exposure to humans of the BSE agent: infective dose and species barrier. Adopted by the SSC at its meeting on the 13th–14th April 2000.
- Stack M, et al. Monitoring for bovine spongiform encephalopathy in sheep in Great Britain, 1998–2004. J. Gen. Virol. 2006;87:2099–2107. doi: 10.1099/vir.0.81254-0. doi:10.1099/vir.0.81254-0 [DOI] [PubMed] [Google Scholar]
- Tongue S.C, Pfeiffer D.U, Warner R, Elliott H, Del Rio Vilas V. Estimation of the relative risk of developing clinical scrapie: the role of prion protein (PrP) genotype and selection bias. Vet. Rec. 2006;158:43–50. doi: 10.1136/vr.158.2.43. [DOI] [PubMed] [Google Scholar]
- van Keulen L.J, Schreuder B.E, Vromans M.E, Langeveld J.P, Smits M.A. Pathogenesis of natural scrapie in sheep. Arch. Virol. Suppl. 2000:57–71. doi: 10.1007/978-3-7091-6308-5_5. [DOI] [PubMed] [Google Scholar]
- van Keulen L.J, Vromans M.E, van Zijderveld F.G. Early and late pathogenesis of natural scrapie infection in sheep. Apmis. 2002;110:23–32. doi: 10.1034/j.1600-0463.2002.100104.x. doi:10.1034/j.1600-0463.2002.100104.x [DOI] [PubMed] [Google Scholar]
- Wells G.A, Hawkins S.A, Green R.B, Austin A.R, Dexter I, Spencer Y.I, Chaplin M.J, Stack M.J, Dawson M. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet. Rec. 1998;142:103–106. doi: 10.1136/vr.142.5.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The model superimposes the spread of BSE upon an age-structured demographic model of sheep in a flock which includes Medelian inheritance of PrP genes. A detailed summary of TSE data used to parameterise the model is also presented