Abstract
It is generally assumed that an individual of a prey species can benefit from an increase in the number of its group's members by reducing its own investment in vigilance. But what behaviour should group members adopt in relation to both the risk of being preyed upon and the individual investment in vigilance? Most models assume that individuals scan independently of one another. It is generally argued that it is more profitable for each group member owing to the cost that coordination of individual scans in non-overlapping bouts of vigilance would require. We studied the relationships between both individual and collective vigilance and group size in Defassa waterbuck, Kobus ellipsiprymnus defassa, in a population living under a predation risk. Our results confirmed that the proportion of time an individual spent in vigilance decreased with group size. However, the time during which at least one individual in the group scanned the environment (collective vigilance) increased. Analyses showed that individuals neither coordinated their scanning in an asynchronous way nor scanned independently of one another. On the contrary, scanning and non-scanning bouts were synchronized between group members, producing waves of collective vigilance. We claim that these waves are triggered by allelomimetic effects i.e. they are a phenomenon produced by an individual copying its neighbour's behaviour.
Keywords: vigilance, anti-predator behaviour, synchronization, allelomimesis, Defassa waterbuck, African antilope
1. Introduction
In prey species, vigilance activity is classically viewed in the context of aggregation that confers protection against predators (Lima 1987; Elgar 1989; Roberts 1996). Several theoretical models have been developed to predict the vigilance behaviour that individual group members should adopt in response to the risk of being preyed upon. The basic model of scanning for predators, developed by Pulliam (1973), relied upon three basic assumptions, as discussed by Bednekoff & Lima (1998). First, predators are assumed to rush from cover at random times. Second, attacking predators that remain undetected for a certain critical time are assumed to be certain of catching their prey. Third, scanning by each group member is assumed to be independent (Pulliam et al. 1982) and interscan intervals are assumed to follow a Poisson distribution. The validity of the model's first two assumptions has been challenged (FitzGibbon 1990; Bednekoff & Lima 1998). The Poisson process, however, has received theoretical support (Scannell et al. 2001). If prey individuals scan randomly, they individually produce a range of long and short bouts of non-vigilance (Hart & Lendrem 1984; Desportes et al. 1989). Thus, assuming a negative exponential distribution of interscan intervals, Scannell et al. (2001) suggested that predators will be unable to predict when randomly scanning prey will next raise their heads, and therefore will be unable to exploit this information in timing their attacks. The main focus of our study is the independence of scanning between group members testing whether individuals act independently of one another, which has previously been assumed in most of theoretical studies of vigilance.
We might suppose that in order to benefit as much as possible from their respective vigilance activities, group members should coordinate their scans in non-overlapping bouts to avoid raising their head (i.e. being vigilant) when another group member is already vigilant. In a theoretical study, Rodríguez-Gironés & Vásquez (2002) show that coordination has a marked positive effect on modelled survival probability. The model seems to show that coordinating anti-predator scans among group members can be more efficient than independent scanning even if individuals must spend a large proportion of their time coordinating their behaviour. However, this phenomenon (e.g. sentinel behaviour) has been reported in only a few species of mammals (Bednekoff 1997; Clutton-Brock et al. 1999) and birds (McGowan & Woolfenden 1989), and seems to be rare in nature (Elcavage & Caraco 1983). The main functional explanation of this non-observation in non-persistent groups is that such coordination is too costly owing to the monitoring of other group members that is needed to achieve coordination (Ward 1985; Lima 1995; see also Ruxton & Roberts 1999). Indeed, to avoid an overlapping of scanning bouts between group members, individuals should continually evaluate the size of the group they are in and adjust their vigilant activities in order to exhibit vigilant acts only when other individuals are not already vigilant. Therefore, although coordination may occasionally be observed in structured gatherings (such as social groups), the individual investment required by each animal suggests that group members should act independently of one another. Thus, independent scanning generally appears to be a good trade-off to achieve efficient collective detection.
From Pulliam's model, theoretical studies have considered whether scanning is independent between group members (Pulliam et al. 1982; see Bednekoff & Lima 1998). However, many observational studies have demonstrated that vigilance of a group member is affected by the presence of neighbours (Bahr & Bekoff 1999), which is especially so for many mammal species (Blumstein et al. 2001; Treves et al. 2001; Cameron & Du Toit 2005). Nevertheless, other studies seem to highlight a possible degree of synchronization when group members scanned their surroundings (Lazarus 1979; Bertram 1980). Although studies have showed synchronization between group members, they did not specially investigate this pattern for prey species under an observed predation risk (Fernández et al. 2003; Pays et al. 2007). Our question was whether the vigilance behaviour of each group member complies with the assumption of independent scanning or whether it influences the timing of vigilant acts and bouts exhibited by other group members. Although independent scanning between group members is commonly assumed in theoretical studies, few studies have investigated the validity of this assumption by observing the behaviour in the wild of prey groups under predation risk.
We studied vigilance activity in a population of Defassa waterbuck, Kobus ellipsiprymnus defassa, which lived under a predation risk in a nature reserve. We considered the two different structural levels at which vigilance can be examined (i.e. individual or group). First, we investigated the relationship between group size and individual vigilance i.e. the time spent by an individual in this activity, and collective vigilance i.e. the time during which at least one individual in the group scans the environment. Second, we tried to determine whether individuals scanned their environment independently of one another (Pulliam's assumption) or were inclined to coordinate their scans in an asynchronous way.
2. Material and methods
(a) Study area and animals
The fieldwork was carried out in the protected area managed by the ECOFAC/PDZCV program in the Central African Republic. The study area, ‘Sangba base’ (Universal Transverse Mercator coordinates: 34N, 754898N, 2055497E), comprises floodplain savannah which offers an exceptional opportunity to observe animals in their natural environment and under natural conditions. We studied groups of Defassa waterbuck, a species which suffers a high predation rate, especially from leopard Panthera pardus (see electronic supplementary material A and Renaud (2006) for more details on the study site). Vigilance activity exhibited by individuals is strongly expected to have an anti-predatory function.
(b) Recording data
We collected behavioural data by videotaping all members of a focal group of waterbuck for a 10 min period (see electronic supplementary material B for details). During sampling, all group members were in the camera's field of view and the group size was recorded. To characterize behaviour, we considered an animal as vigilant when it did not move its feet and raised its head above horizontal, scanning its surroundings. No ambiguities were encountered in distinguishing a vigilant from a non-vigilant animal. For analysis, video sequences were converted to analytic sequences. For each individual within each group, a binary sequence (0, non-vigilance activity and 1, vigilance activity) was constructed reflecting its activity state precisely at each second for 600 s. We recorded the activity of each group member at precisely the same time, and thereby quantified the individual and collective levels of vigilance with the methods described below. In this way, 197 individuals corresponding to 46 groups were sampled.
(c) Data analyses
From the analytic sequences, we calculated for each individual within each group the means of the length of its scan duration and of its anti-scan duration (i.e. the time between the end of one and the beginning of the next vigilant event), in seconds, as well as the number of vigilant acts it performed during the 10 min sequence. The three variables, scan duration, anti-scan duration and the frequency of vigilant acts per minute, were ln transformed. For each individual in each group, the individual vigilance was also characterized by the proportion of time (Pind) the individual spent in vigilance and logit transformed: logit (Pind)=ln (Pind/(1−Pind)). We investigated the relationship between group size and the four transformed individual variables, using the linear mixed-effects model fitted by REML and introducing a random effect due to the presence of groups. In this method it is crucial to analyse individual variables (N=197), taking the 46 groups into account.
At each second, the video-recorded activities of all group members allowed us to identify when at least one member of the group scanned the environment (i.e. whether there was collective vigilance). The duration and the frequency of bouts of collective vigilance were calculated, as well as the observed proportion of time (Pobs) for which the group showed collective vigilance. Only groups containing at least two individuals (N=42) were used for the analysis of group vigilance.
We used the same procedure to test whether individuals tended to scan their environment independently of one another or tended to coordinate their scans in non-overlapping bouts. We compared the observed collective vigilance to the expected proportion of time during which at least one member of the group would have scanned the environment under an assumption of independent scanning.
This expected proportion was estimated by , where pk was the proportion of time the individual k spent in vigilance, and n, the group size (Fernández et al. 2003; Pays et al. 2007). After a logit transformation, observed and expected proportions were compared with a Student's t-test for paired samples. If vigilance behaviours of individuals in the same group occurred independently of one another, the difference between observed and expected proportions of time would not differ statistically from 0. However, if individuals tended to coordinate their scans in non-overlapping bouts, the difference would be significantly greater than 0. It would also mean that values less than 0 suggest synchronized vigilance.
If the n group members acted independently of one another and spent the same proportion of time q in vigilance, the proportion of time during which at least one group member scanned should be Pobs=1−(1−q)n (see the formula for Pexp in the previous paragraph). On this basis, from the observed proportion of time (Pobs) for which the group showed collective vigilance, we computed an intermediate variable q=1−(1−Pobs)1/n, where n is the group size. This variable q was logit transformed and the group-size effect was tested using a linear model. Expected functions linking group size and the duration and frequency of bouts of collective vigilance and the duration of intervals between two successive bouts of collective vigilance were much more difficult to determine. Thus, group-size effect was tested on these three variables simply using a Spearman's rank correlation with non-transformed data.
From each observed group, we calculated a Pearson's correlation coefficient between two binary sequences (0 for non-vigilant and 1 for vigilant activity) of two different individuals within the group. This coefficient was calculated for all of the possible pairs of group members. Then, we calculated the mean of the correlation coefficients for each observed group and compared it to the mean of the correlation coefficients expected under the assumption that individuals scan independently of one another (see the method based on simulation, electronic supplementary material C). The observed and simulated means of correlation coefficients were compared using a Wilcoxon T test for paired samples. If individuals tend to act independently of one another, the observed and expected means would not be statistically different. If individuals tend to coordinate their bouts of vigilance, the observed mean would be significantly lower than the expected; whereas if individuals tend to synchronize their vigilant bouts, the observed mean would be significantly higher than the expected.
3. Results
(a) Individual vigilance
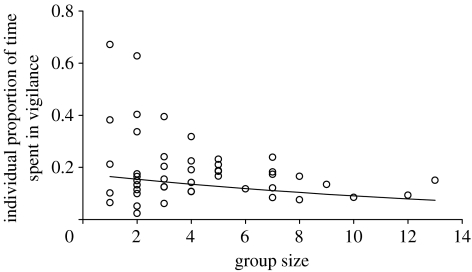
Mixed linear models computed on the transformed data showed that the mean duration of vigilant acts of an individual tended to decrease when group size increased (coef.±s.e.=−0.029±0.015; F1,44=3.784; p=0.058), while the mean duration between two successive vigilant acts (anti-scan duration) strongly increased (coef.±s.e.=0.052±0.022; F1,44=5.512; p=0.023). Although the number of acts per minute for an individual within a group did not significantly decrease with the group size (coef.±s.e.=−0.032±0.019; F1,44=2.741; p=0.105), the proportion of time that an individual spent in vigilance decreased (figure 1).
Figure 1.
Group-size effect on individual vigilance i.e. the proportion of time (Pind) that an individual spent in vigilance within a group (Y=exp(−1.548−0.076X)/[1+exp(−1.548−0.076X)]; F1,44=7.131; p=0.011). Each dot represents the mean value for the group but the linear regression was calculated between group size and the logit-transformed proportion of time using individual values and including a ‘group’ random effect in the linear mixed-effects model (see §2).
(b) Collective pattern of vigilance
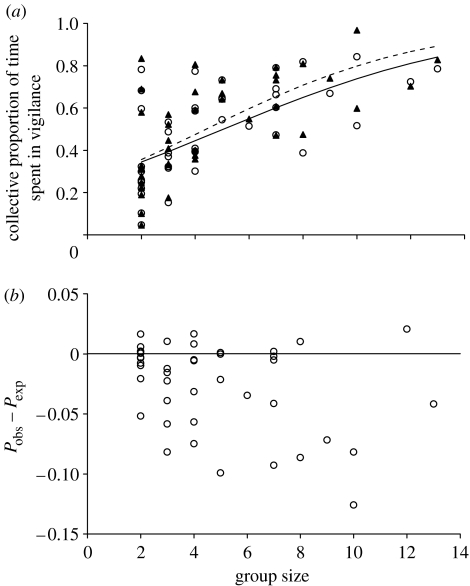
While the duration of collective vigilance bouts increased with group size (Spearman's rank correlation rS=0.353; N=42; p=0.025), duration of interval between two successive bouts of collective vigilance decreased very significantly as group size increased (rS=−0.556; N=42; p=0.0004) and the frequency of bouts of collective vigilance increased strongly with group size (rS=0.495; N=42; p=0.002). Finally, the collective proportion of time during which at least one individual was vigilant increased very significantly (figure 2a).
Figure 2.
(a) Group-size effect on the observed collective proportion and the expected proportion under the independent scanning assumption. Open circles indicate the observed proportions in each group (Pobs) i.e. the proportion of time that at least one individual spent in vigilance within a group and the solid-line, the observed linear regression on the logit-transformed Pobs: Y=1−[1/(1+exp(−1.06+0.210X))X]; F1,40=21.577; p<0.0001). Filled triangles indicate the expected values of proportion of time and the dotted-line, the expected linear regression under the independent scanning assumption (linear regression on the logit-transformed Pexp: Y=1−[1/(1+exp(−1.086+0.246X))X]; F1,40=24.697; p<0.0001). (b) Difference between the observed collective proportion (Pobs) and the expected collective proportion of time (Pexp) under an assumption of independent scanning, for each observed group.
The difference between the observed proportion of time for which the group showed collective vigilance and the proportion expected under the assumption that the individuals within a group scan their environment independently of one another was significantly different from 0 (Student's t-test for paired samples: t41=−4.616; p<0.0001; figure 2b). Thus, individuals within groups did not scan their environment independently of one another. Moreover, the observed proportion was most often less than that expected under an assumption of independent scanning. Therefore, individuals do not seem to coordinate their scanning in an asynchronous way but rather seem to synchronize their individual vigilance activity.
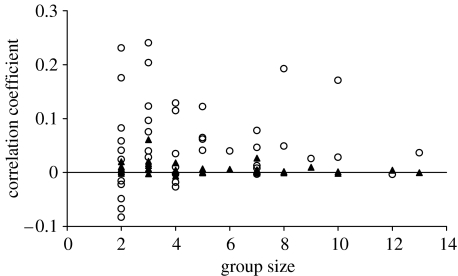
Within each group, the mean of the observed correlation coefficients between two individuals in groups, considering all possible association pairs, is much higher than that expected under the assumption that individuals scan independently of one another (Wilcoxon test for paired samples: T=755; N=42; p=0.0001; figure 3). Thus, bouts of individual vigilant and non-vigilant activity tend to be synchronized between group members within groups, producing waves of collective vigilance.
Figure 3.
For each observed group, an open circle is the mean of the observed correlation coefficient between each pair of group members and a filled triangle is the expected value under the assumption that they scan their environment independently of one another (see §2).
4. Discussion
As we might expect, our results support models of vigilance derived from Pulliam's original prediction (1973) that members benefit from grouping by reducing the amount of time spent in individual vigilance, while increasing the time spent feeding, without affecting their probability of detecting a predator. Although few studies have quantified collective vigilance, some revealed that this collective activity increased with group size (Bertram 1980), while others showed that the proportion of time when at least one group member was vigilant did not vary with the group size (Fernández et al. 2003). In the Defassa waterbuck, we found that collective vigilance continuously increased with the group size to reach a high proportion of time. However, it should be noted that a continuous increase in collective vigilance with group size is not necessarily to be expected since in a large group an individual benefits not only from the vigilance activity of the other group members but also from a dilution effect (Lima 1995). Therefore, at the first sight, irrespective of the pattern of collective vigilance produced by Defassa waterbuck, it seems to be relatively efficient for group members because it clearly increases the likelihood of collective detection of a temporally unpredictable predator.
Our results show that group members living under a predation risk do not organize their behaviours (vigilance versus foraging) through coordination i.e. with non-overlapping bouts of vigilant activity. Indeed, our results support evidence for the rarity of this expected advantage in nature (Elcavage & Caraco 1983). Likewise, our results do not support the assumption of independent scanning by group members. On the contrary, group members tend to scan at the same time more frequently than expected by chance. Therefore, individuals seem to synchronize their vigilant bouts and waves of collective vigilance emerge. This collective pattern has also been found in other species such as greater rhea, Rhea americana, a large flightless bird that inhabits open areas (Fernández et al. 2003). Although most theoretical models of vigilance assume independent scanning (Bednekoff & Lima 1998), we suggest that this assumption should be tested in other species.
From a functional point of view, some synchronization of behaviour between members within groups is essential for group cohesion (Engel & Lamprecht 1997), and such cohesion may be advantageous for group members (Hamilton 1971; Pulliam 1973). Elgar et al. (1984) and Lima (1995) suggested that animals respond to an attack more quickly when they were already alerted than when they were feeding. But, being synchronized with other group members for vigilance and for feeding is certainly not optimum to detect predators because this pattern results in time in which all individuals are feeding and none are vigilant.
Two different mechanisms could generate the observed synchronization of vigilance among group members. Synchronization might be caused by stimuli from the environment. Under this assumption, all group members might raise their heads because all individuals detected the same disturbance, outside the group, at the same time. For example, a detected predator that is about to attack or is actually attacking would alert all group members and would induce a bout of collective vigilance. Nevertheless, it seems unlikely that external stimuli were the main cause of synchronization in our case because the mechanism that produces this collective pattern also generates a clear correlation of individual and collective vigilance with group size. Therefore, we strongly suggest an intrinsic cause to the group vigilance and one that would thus be group-size dependent. One individual might raise its head, alerting other group members that would then perform a similar vigilant act (raise their heads) without having seen the source of disturbance themselves. In this case, successive head-up acts may progressively emerge in the group characterized by the contagious effect triggered by individuals copying the alerted initiator's behaviour. Indeed, when an individual detects a predator, this information may be passed to its group mates either through an alarm call or the sudden departure of that individual (Pulliam 1973; Davis 1975; Lazarus 1979). In the same way, waves of collective vigilance may be triggered by a copying phenomenon as an allelomimetic effect, i.e. a phenomenon that consists of an individual copying its neighbour's behaviour (Deneubourg & Goss 1989; Parrish & Edelstein-Keshet 1999). Quenette & Gerard (1992) found that wild boar, Sus scrofa, individuals tend to copy the feeding behaviour of neighbours. It would not be surprising to find that allelomimetic effects exist for multiple activities within a group. However, more studies are needed on this phenomenon.
Our study not only confirms the expected negative relationship between an individual waterbuck's time spent vigilant and the size of group in which it occurs, but also demonstrates the strongly positive relationship between collective vigilance and group size. Thus, an individual waterbuck that is a member of a coherent and communicating group enjoys dual benefits: it can reduce time spent vigilant, while also enjoying greater security through an increased probability that at least one waterbuck will be vigilant at any moment. The demonstrated synchrony in vigilance implies that a non-trivial proportion of an individual's vigilance coincides with and may occur in response to the vigilance of other group members. This further suggests that the effects of group membership (i.e. the emergence of the collective pattern of vigilance) should be considered in new models of vigilance.
Acknowledgments
We are grateful to M. Goulard for the insight into statistical analyses. We thank A. Abdoulaye for the help in filming animals. We are grateful to ECOFAC/PDZCV and AGRECO, in particular R. Mbitikon and F. Feys who allowed us to work in the Sangba base.
Supplementary Material
Appendix A: study site. Appendix B: animal sample, details concerning the conditions to film animals. Appendix C: correlation coefficient method, details of the method to simulate the correlation coefficient expected under the assumption that individuals scan independently of one another
References
- Bahr D.B, Bekoff M. Predicting flock vigilance from simple passerine interactions: modelling with cellular automata. Anim. Behav. 1999;58:831–839. doi: 10.1006/anbe.1999.1227. doi:10.1006/anbe.1999.1227 [DOI] [PubMed] [Google Scholar]
- Bednekoff P.A. Mutualism among safe, selfish sentinels: a dynamic game. Am. Nat. 1997;150:373–392. doi: 10.1086/286070. doi:10.1086/286070 [DOI] [PubMed] [Google Scholar]
- Bednekoff P.A, Lima S.L. Randomness, chaos and confusion in the study of antipredator vigilance. Trends Ecol. Evol. 1998;13:284–287. doi: 10.1016/s0169-5347(98)01327-5. doi:10.1016/S0169-5347(98)01327-5 [DOI] [PubMed] [Google Scholar]
- Bertram B.C.R. Vigilance and group size in ostriches. Anim. Behav. 1980;28:278–286. doi:10.1016/S0003-3472(80)80030-3 [Google Scholar]
- Blumstein D.T, Daniel J.C, McLean I.G. Group size effects in quokkas. Aust. J. Zool. 2001;49:641–649. doi:10.1071/ZO01032 [Google Scholar]
- Cameron E, Du Toit J.T. Social influences on vigilance behaviour in giraffes, Giraffa camelopardalis. Anim. Behav. 2005;69:1337–1344. doi:10.1016/j.anbehav.2004.08.015 [Google Scholar]
- Clutton-Brock T.H, O'Riain M.J, Brotherton P.N.M, Gaynor D, Kansky R, Griffin A.S, Manser M. Selfish sentinels in cooperative mammals. Science. 1999;284:1640–1644. doi: 10.1126/science.284.5420.1640. doi:10.1126/science.284.5420.1640 [DOI] [PubMed] [Google Scholar]
- Davis J.M. Socially induced flight reactions in pigeons. Anim. Behav. 1975;23:597–601. doi:10.1016/0003-3472(75)90136-0 [Google Scholar]
- Desportes J.-P, Metcalfe N.B, Cézilly F, Lauvergeon G, Kervelle C. Tests of the sequential randomness of vigilant behaviour using spectral analysis. Anim. Behav. 1989;38:771–777. doi:10.1016/S0003-3472(89)80109-5 [Google Scholar]
- Deneubourg J.-L, Goss S. Collective patterns and decision-making. Ethol. Ecol. Evol. 1989;1:295–311. [Google Scholar]
- Elcavage P, Caraco T. Vigilance behaviour in house sparrow flocks. Anim. Behav. 1983;31:303–304. doi:10.1016/S0003-3472(83)80200-0 [Google Scholar]
- Elgar M.A. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. 1989;64:13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Elgar M.A, Burren P.J, Posen M. Vigilance and perception of flock size in foraging house sparrows (Passer domesticus) Behaviour. 1984;90:215–223. [Google Scholar]
- Engel J, Lamprecht J. Doing what everybody does? A procedure for investigating behavioural synchronization. J. Theor. Biol. 1997;185:255–262. doi: 10.1006/jtbi.1996.0359. doi:10.1006/jtbi.1996.0359 [DOI] [PubMed] [Google Scholar]
- Fernández G.J, Capurro A.F, Reboreda J.C. Effect of group size on individual and collective vigilance in greater rheas. Ethology. 2003;109:413–425. doi:10.1046/j.1439-0310.2003.00887.x [Google Scholar]
- FitzGibbon C.D. Mixed-species grouping in Thomson's gazelles: the antipredator benefits. Anim. Behav. 1990;40:837–845. doi:10.1016/S0003-3472(05)80984-4 [Google Scholar]
- Hamilton W.D. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. doi:10.1016/0022-5193(71)90189-5 [DOI] [PubMed] [Google Scholar]
- Hart A, Lendrem D.W. Vigilance and scanning patterns in birds. Anim. Behav. 1984;32:1216–1224. doi:10.1016/S0003-3472(84)80239-0 [Google Scholar]
- Lazarus J. The early warning function of flocking in birds: an experimental study with captive quelea. Anim. Behav. 1979;27:855–865. doi:10.1016/0003-3472(79)90023-X [Google Scholar]
- Lima S.L. Vigilance while feeding and its relation to the risk of predation. J. Theor. Biol. 1987;124:303–316. doi:10.1016/S0022-5193(87)80118-2 [Google Scholar]
- Lima S.L. Back to the basics of anti-predatory vigilance: the group-size effect. Anim. Behav. 1995;49:11–20. doi:10.1016/0003-3472(95)80149-9 [Google Scholar]
- McGowan K.J, Woolfenden G.E. A sentinel system in the Florida scrub jay. Anim. Behav. 1989;37:1000–1006. doi:10.1016/0003-3472(89)90144-9 [Google Scholar]
- Parrish J.K, Edelstein-Keshet L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science. 1999;284:99–101. doi: 10.1126/science.284.5411.99. doi:10.1126/science.284.5411.99 [DOI] [PubMed] [Google Scholar]
- Pays, O., Jarman, P., Loisel, P. & Gerard, J.-F. 2007 Coordination, independence or synchronisation tendency of individual vigilance in the eastern grey kangaroo? Anim. Behav. (doi:10.1016/j.anbehav.2006.06.007)
- Pulliam H.R. On the advantages of flocking. J. Theor. Biol. 1973;38:419–422. doi: 10.1016/0022-5193(73)90184-7. doi:10.1016/0022-5193(73)90184-7 [DOI] [PubMed] [Google Scholar]
- Pulliam H.R, Pyke G.H, Caraco T. The scanning behaviour of juncos: a game–theoretical approach. J. Theor. Biol. 1982;95:89–103. doi:10.1016/0022-5193(82)90289-2 [Google Scholar]
- Quenette P.Y, Gerard J.F. From individual to collective vigilance in wild boars (Sus scrofa) Can. J. Zool. 1992;70:1632–1635. [Google Scholar]
- Renaud, P. C. 2006 Recensement aérien de la faune dans les préfectures de la région Nord de la République Centrafricaine. Rapport de mission FCT 2005. IUE/DCE Bangui.
- Roberts G. Why individual vigilance declines as group size increases. Anim. Behav. 1996;51:1077–1086. doi:10.1006/anbe.1996.0109 [Google Scholar]
- Rodríguez-Gironés M.A, Vásquez R.A. Evolutionary stability of vigilance coordination among social foragers. Proc. R. Soc. B. 2002;269:1803–1810. doi: 10.1098/rspb.2002.2043. doi:10.1098/rspb.2002.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton G.D, Roberts G. Are vigilance sequences a consequence of intrinsic chaos or external changes? Anim. Behav. 1999;57:493–495. doi: 10.1006/anbe.1998.0965. doi:10.1006/anbe.1998.0965 [DOI] [PubMed] [Google Scholar]
- Scannell J, Roberts G, Lazarus J. Prey scan at random to evade observant predators. Proc. R. Soc. B. 2001;268:541–547. doi: 10.1098/rspb.2000.1388. doi:10.1098/rspb.2000.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Drescher A, Ingrisano N. Vigilance and aggregation in black howler monkeys (Alouatta pigra) Behav. Ecol. Sociobiol. 2001;50:90–95. doi:10.1007/s002650100328 [Google Scholar]
- Ward P. Why birds do not coordinate their vigilance? J. Theor. Biol. 1985;114:383–385. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A: study site. Appendix B: animal sample, details concerning the conditions to film animals. Appendix C: correlation coefficient method, details of the method to simulate the correlation coefficient expected under the assumption that individuals scan independently of one another