Abstract
The Indian gharial (Gavialis gangeticus) is not found in saltwater, but the geographical distribution of fossil relatives suggests a derivation from ancestors that lived in, or were at least able to withstand, saline conditions. Here, we describe a new Oligocene gharial, Aktiogavialis puertoricensis, from deltaic–coastal deposits of northern Puerto Rico. It is related to a clade of Neogene gharials otherwise restricted to South America. Its geological and geographical settings, along with its phylogenetic relationships, are consistent with two scenarios: (i) that a single trans-Atlantic dispersal event during the Tertiary explains the South American Neogene gharial assemblage and (ii) that stem gharials were coastal animals and their current restriction to freshwater settings is a comparatively recent environmental shift for the group. This discovery highlights the importance of including fossil information in a phylogenetic context when assessing the ecological history of modern organisms.
Keywords: biogeography, Crocodylia, Gavialis, gharial, Aktiogavialis, phylogeny
1. Introduction
Gavialis gangeticus (the Indian gharial) is a large crocodylian with a long, tubular snout often thought to reflect a specialization for catching fishes (Langston 1973). Its historical distribution is limited to inland drainages of the Indian subcontinent (Smith 1933; Singh 1991). Although there are anecdotal reports of its occurrence in seasonally brackish bodies of water (Annandale 1915), it favours deep rivers and is viewed as a freshwater reptile. It is also thought to be the most aquatic living crocodylian with limited overland dispersal capacity (Whitaker & Basu 1983). One might thus expect a distribution for Gavialoidea (G. gangeticus and all crocodylians closer to it than to Alligator mississippiensis or Crocodylus niloticus; Brochu 2003), either limited in geographical scope or dominated by vicariance.
Although many Neogene fossil gavialoids occur in non-marine depositional systems (e.g. Janensch 1911; Lull 1944; Langston 1965; Langston & Gasparini 1997), their post-Oligocene geographical distribution is difficult to reconcile with a vicariance model. They appear in South America during the Miocene with no known close relatives in North America, suggesting dispersal from Africa or, less probably, Asia (Buffetaut 1982; Langston & Gasparini 1997; Brochu & Rincon 2004). Vicariance explains this distribution only if derived gavialoids have unsampled histories on multiple continents extending from the Miocene back to the Mesozoic.
Nearly all reported gavialoids from the Late Cretaceous through the Palaeocene—the so-called ‘thoracosaurs’—are from shallow marine or marginal marine deposits (e.g. Köken 1888; Troedsson 1924; Piveteau 1927; Gallagher et al. 1986; Schwimmer 1986; Erickson 1998; Zarski et al. 1998; Weems 1999; Laurent et al. 2000; Brochu 2004, 2006; Hua & Jouve 2004; Robb 2004; Delfino et al. 2005). This might support transoceanic dispersal as an important factor in explaining gavialoid distribution, but these are controversial owing to the debate over the relationships and divergence timing of extant Gavialis. Parsimony analyses of morphology support a clade including thoracosaurs and more derived gavialoids at the base of Crocodylia, with a divergence time between Gavialis and any other living crocodylian minimally in the Campanian (Norell 1989; Salisbury & Willis 1996; Brochu 1997, 2003; Hua & Jouve 2004; Jouve et al. 2006). Molecular data consistently support a close phylogenetic relationship between Gavialis and another freshwater-restricted crocodylian—the Indonesian false gharial (Tomistoma schlegelii)—and the divergence time between them within the Tertiary (Densmore 1983; Densmore & Owen 1989; Hass et al. 1992; White & Densmore 2001; Gatesy et al. 2003; Harshman et al. 2003; Janke et al. 2005; McAliley et al. 2006). If molecular data are correct with respect to divergence time, thoracosaurs are not gavialoids (Harshman et al. 2003) and are irrelevant to the debate surrounding the ecology and physiology of extinct relatives of Gavialis.
We describe a new fossil gavialoid from the Oligocene San Sebastián Formation of Puerto Rico (figure 1) closely related to extinct gavialoids from the Mio–Pliocene of South America. Although relationships among derived gavialoids are ambiguous about the biogeographic origin of the Neotropical clade, the presence of a member in the Greater Antilles during the Oligocene supports an African origin with a single trans-Atlantic dispersal event. The Puerto Rican gharial and other South American gharials are from marginal marine deposits which suggests that the ability to live in, or at least withstand, saltwater was typical among gavialoids until comparatively recently.
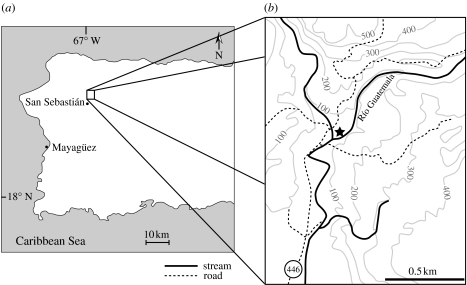
Figure 1.
(a) Map of the western half of Puerto Rico showing the location of the town of San Sebastián. (b) Geologic map of the locality where UPRMP 3094 was collected. Star indicates the site where holotype was collected. Contour interval=100 m.
2. Systematic palaeontology
CROCODYLIA Gmelin 1789 (sensu Benton & Clark 1988)
GRYPOSUCHINAE tax. nov.
GAVIALOIDEA Hay 1930 (sensu Brochu 2003)
Aktiogavialis puertoricensis tax. nov.
(a) Etymology
Aktios, Gr., from the shore or beach; gavialis, gharial; puertoricensis, Puerto Rico.
(b) Holotype
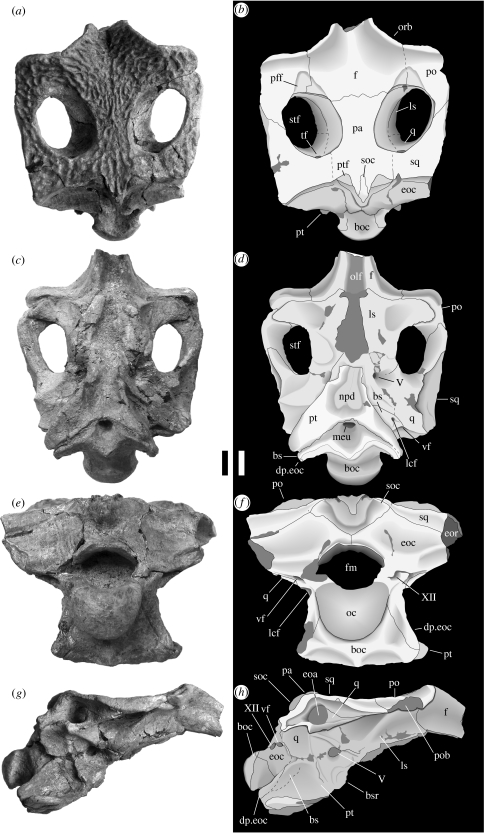
University of Puerto Rico Department of Geology Paleontology Collection, Mayagüez (UPRMP) 3094, incomplete skull including the braincase and skull table behind the orbits (figure 2).
Figure 2.
UPRMP 3094, holotype, Aktiogavialis puertoricensis: (a,b) dorsal view, (c,d) ventral view, (e,f) posterior view, and (g,h) right lateral view. Scale bar, 1 cm. boc, basioccipital; bs, basisphenoid; bsr, base of basisphenoid rostrum; dp.eoc, descending process of exoccipital; eoa, external otic aperture; eoc, exoccipital; eor, external otic recess; f, frontal; fm, foramen magnum; lcf, lateral carotid foramen; ls, laterosphenoid; meu, median Eustachian foramen; npd, roof of nasopharyngeal duct; oc, occipital condyle; olf, pathway of olfactory tract; orb, orbit; pa, parietal; pff, fossa anterior to supratemporal fenestra; po, postorbital; pob, base of postorbital bar; pt, pterygoid; ptf, post-temporal fenestra; q, quadrate; soc, supraoccipital; sq, squamosal; stf, supratemporal fenestra; tf, temporal foramen; V, trigeminal foramen; vf, vagus foramen; XII, aperture for 12th cranial nerve.
(c) Locality and horizon
The holotype was collected from a medium- to coarse-grained sandstone in the lower San Sebastián Formation exposed along Río Guatemala near the town of San Sebastián (18°21′24.6″ N, 66°59′41.8″ W; figure 1). Exposures in the area consist of soils interbedded with marine mudstones and siltstones grading up into deltaic sands, all of which are part of the basal San Sebastián Formation (Ward et al. 2002). Additional vertebrate remains from the section include elasmobranchs, turtles, sirenians and rodents (MacPhee & Wyss 1990; J. V. J. & H.S. unpublished data).
Biostratigraphically informative ostracodes from mudstones in the collection area indicate an Oligocene age for the section (van den Bold 1965). Strontium isotopic ages (Johnson et al. 2006; Ramirez et al. 2006) and biostratigraphic evidence from planktic foraminifera and calcareous nannofossils (Monroe 1980; Frost et al. 1983; Seiglie & Moussa 1984) from the lower part of the overlying Lares Limestone unambiguously place the San Sebastián–Lares contact within the Upper Oligocene, further indicating a pre-Miocene age for the holotype.
An isolated crocodylian caudal vertebra was collected from the same horizon as the holotype. Other crocodylian remains collected from stratigraphically lower units at this locality consist of vertebrae and scutes similar to those from other San Sebastián localities (Brochu et al. 2007).
(d) Diagnosis
Gavialoid crocodylian with a distinct fossa extending anteriorly from the supratemporal fenestra on the dorsal postorbital surface. Absence of prootic exposure on the lateral braincase wall with at least some gryposuchines. Supratemporal fenestrae are larger anteroposteriorly than mediolaterally (could reflect ontogenetic variation).
(e) Description
Although incomplete, the preserved portions are undistorted (figure 2). Water abrasion obscures some sutures.
The margins of the orbits are upturned medially and posteriorly, giving them the ‘telescoped’ appearance typical of more derived gavialoids. The dorsal surfaces of the fused frontals are concave between the orbits and bear an anteroposteriorly oriented groove on the ventral surface for the olfactory tract. The postorbital bars are not preserved, but the ventral surfaces of the postorbitals indicate where they descended from the skull table and indicate robust bars.
The skull table is dorsally reflected anteriorly, and the postorbitals form a pair of arches where they form the orbital margins. The supratemporal fenestrae are subcircular with long axes oriented anterolaterally. The frontoparietal suture intersects the fenestral margins, but the parietal and postorbitals maintain robust contacts within the fenestral space. Within the supratemporal fenestrae, the quadrate forms the ventral margin of the circular temporal foramen.
A distinct fossa extends anteriorly from the supratemporal fenestra on the dorsal postorbital surface (figure 2a,b). The floor of the fossa is pitted and the posterior limit corresponds with the postorbital–parietal suture. This feature is unknown in any other crocodylian.
The posterior margin of the skull table projects posteriorly and forms an acute process. The supraoccipital is exposed dorsally as a diamond-shaped element at the posteriormost tip of this process adjacent to the parietal. The dorsal surfaces of the post-temporal fenestrae appear not to have extended back to the posteriormost tip of this process.
The squamosal bears an indistinct, but anteriorly flaring, lateral groove for attachment of the ear flap musculature (figure 2g,h). The squamosal is dorsoventrally very thin immediately dorsal to the triangular external otic aperture. Within the otic recess, the squamosal forms the posterior and dorsal margins of the aperture, and the squamosal–quadrate suture intersects the apertural margin at its posteroventral corner.
Portions of the lateral braincase wall are damaged, making it difficult to trace some sutures. However, there is no trace of the prootic on either lateral braincase wall, and the surface surrounding the trigeminal foramen (where the suture would be expected in a gavialoid) is relatively well preserved on both sides (figure 2g,h). A notch on the laterosphenoid extending anteriorly from the trigeminal foramen probably indicates the pathway of the ophthalmic branch of the trigeminal nerve. A shallow fossa anteroventral to the trigeminal foramen constricts the braincase where the lost basisphenoid rostrum would have attached. The posterior half of the lateral braincase wall is concave behind a thin dorsoventral crest on the surfaces of the quadrate and pterygoid.
The laterosphenoid capitate processes meet the ventral surface of the skull table with anterolaterally oriented anterior margins (figure 2c,d). Most of the pterygoid is missing, but the dorsal surface of the nasopharyngeal duct is preserved, suggesting a posteroventrally oriented aperture with a modest dorsal septum. The medial Eustachian foramen is large and encircled by the basisphenoid, which is exposed as an anteroposteriorly broad element between the basioccipital and pterygoid.
In posterior view (figure 2e,f), the dorsal margin of the skull table slopes ventrally lateral to the parietal. The exoccipitals meet at the midline dorsal to the foramen magnum and bear broad descending processes that continue to the ventral surface of the basioccipital tubera. The vagus foramen is an oval slit projecting posteroventrally, and the lateral carotid foramen is a circular opening immediately ventral to the vagus foramen. A single foramen for the accessory nerve is preserved on the right side. Since the basisphenoid suture is not preserved on the lateral braincase surface, the relationship between the lateral exposure of the basisphenoid and lateral carotid foramen cannot be ascertained. The right posterior pterygoid process is a small, posteriorly projecting element lateral to the basioccipital tuber. The lateral Eustachian foramina are not preserved, but the ventral margin of the basioccipital tubera is nearly horizontal in posterior view, with deep posterior concavities between the sagittal crest and lateral sides, suggesting a placement lateral to the median Eustachian foramen.
3. Phylogenetic analysis
(a) Methods
Aktiogavialis was added to a matrix of 166 morphological characters (Brochu 2004, 2006; Brochu & Rincon 2004; see electronic supplementary material). The matrix included 57 ingroup taxa including Aktiogavialis. The putative gavialoid Piscogavialis jugaliperforatus (Kraus 1998) from the Miocene of Peru was added based on codings published by Delfino et al. (2005) with modifications from direct observation of the type. Bernissartia fagesii and Hylaeochampsa vectiana were designated as sequential outgroups.
The matrix was subjected to a maximum parsimony analysis using PAUP* v. 4.0b10 (Swofford 2002). One hundred heuristic searches were performed with the starting order of ingroup taxa randomized in each search. Bootstrap support, based on 100 000 replicate datasets, was assessed both globally (for the complete set of taxa used in the parsimony analysis) and locally (for a dataset restricted to the gavialoids, with Borealosuchus sternbergii and H. vectiana used as outgroups).
(b) Results
The analysis recovered 289 446 equally optimal trees, all but one within a single parsimony island (length=449, CI without uninformative characters=0.424, RI=0.785). The strict consensus topology (figure 3) is consistent with previous analyses, with a monophyletic Gavialoidea at the root of Crocodylia and Tomistominae nested deeply within Crocodyloidea.
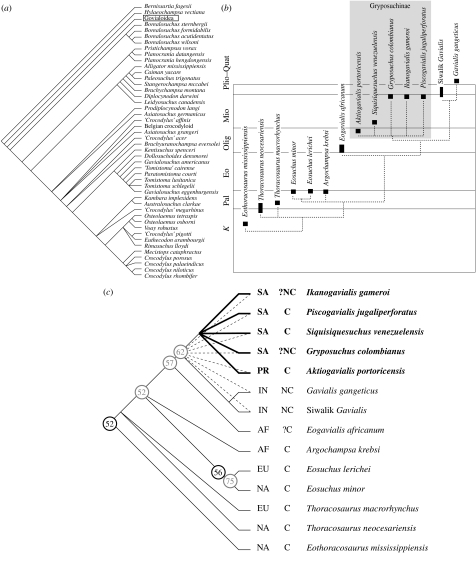
Figure 3.
(a) Strict consensus of 289 446 equally optimal trees from maximum parsimony analysis; Gavialoidea collapsed. (b) Phylogenetic relationships among gavialoids in this analysis shown in stratigraphic position. (c) Phylogenetic relationships among gavialoids; gryposuchines are shown in boldface. Numbers in circles are bootstrap proportions for full (black) and restricted (grey) analyses (see text). Dashed lines indicate loss of resolution if condition of prootic in Aktiogavialis is coded as unknown. Columns between tree and taxon names indicate geographical occurrence (left) and whether or not taxon is from a coastal (C) or non-coastal (NC) setting. AF, Africa; EU, Europe; IN, Indian subcontinent; NA, North America; PR, Puerto Rico; SA, South America.
Based on these results, A. puertoricensis is a derived gavialoid belonging to a group otherwise occurring in the Mio–Pliocene of South America: P. jugaliperforatus, Siquisiquesuchus venezuelensis (Brochu & Rincon 2004); Gryposuchus colombianus (Langston 1965); and Ikanogavialis gameroi (Sill 1970). This group is closely related to extant Gavialis and Miocene Gavialis from the Indian subcontinent.
Bootstrap support is comparatively low (figure 3), and trees one step longer than optimal cause relationships within the clade including Gavialis and the South American forms to collapse. Within the South American clade, all placements of Aktiogavialis are equally optimal except as the sister taxon to Ikanogavialis or Piscogavialis (each of which would increase tree length by one step).
4. Discussion
(a) Nomenclature
We define Gryposuchinae as a stem-based group name including Gryposuchus jessei (Gürich 1912) and all crocodylians closer to it than to G. gangeticus or T. schlegelii. We acknowledge the relatively low support Gryposuchinae currently receives, but we expect this to grow as new characters are added to the analysis and more complete fossils are described.
(b) Phylogenetic relationships
Until recently, the Tertiary record of crocodylians in Puerto Rico was limited to fragmentary material that could not be diagnosed to any particular crocodylian lineage (MacPhee & Wyss 1990). However, isolated osteoderms and a left quadrate ramus were consistent with corresponding parts from gavialoids. In particular, the osteoderms were rectangular, concave anteriorly in dorsal view and had small keels closer to the posterior margin than the anterior. This latter feature is typical of derived gavialoids, including Neogene gryposuchines (Brochu et al. 2007).
Although quantitative support for the position of Aktiogavialis among gavialoids is low, supporting characters are compelling. The descending processes of the exoccipitals lateral to the occipital condyle are anteroposteriorly broad and extend to the ventral surface of the basioccipital tubera, a feature found exclusively in gavialoids. The basisphenoid is not exposed as a broad sheet ventral to the median Eustachian foramen, and it shares with Eosuchus and more derived forms an anteroposteriorly broad basisphenoid adjacent to the median Eustachian aperture. The orbital margin is telescoped, as are those of Eogavialis, Gavialis and other gryposuchines. With Gavialis and gryposuchines, Aktiogavialis shares small, posteriorly oriented posterior pterygoid processes.
Gryposuchines share a lack of a broad prootic exposure around the trigeminal foramen on the lateral braincase wall. Most gavialoids retain the plesiomorphic condition for Crocodylia, in which the prootic can be seen on the lateral braincase wall partially surrounding the trigeminal foramen. Three gavialoids—Aktiogavialis, Gryposuchus and Piscogavialis—lack this exposure (Langston & Gasparini 1997; Brochu & Rincon 2004; C. A. Brochu 1995, 1997; personal observations). This condition also occurs independently among derived alligatoroids and crocodyloids. The condition of the prootic is unknown in Ikanogavialis and Siquisiquesuchus. Other characters supporting gryposuchine monophyly (Brochu & Rincon 2004) are unknown in Aktiogavialis.
Water abrasion complicates this interpretation. Sutures are difficult to trace on the external surface, and the absence of a lateral prootic exposure could reflect non-preservation. If we recode the character expressing exposure of the prootic as unknown in Aktiogavialis, it can equally parsimoniously be made the sister species to any of the derived gavialoids, resulting in the collapse of the South American clade and Gavialis (figure 3). Discovery of additional material would clarify the matter.
Another complication is possible immaturity of the specimen. Differences in the shape of the supratemporal fenestrae, and in the extent to which the frontoparietal suture intersects the fenestral margins, are ontogenetically variable in extant crocodylians. Specific allometric changes vary among taxa, but in extant Gavialis the supratemporal fenestra grows comparatively wider, constricting the interfenestral bar in older animals and incorporating a frontoparietal suture that, in earlier ontogenetic stages, lies entirely on the dorsal skull table surface (Kälin 1933; Brochu 2004).
The shape of the supratemporal fenestrae approximates that of immature Gavialis—the long axis is oriented anterolaterally and diverges from the sagittal plane by approximately 25°. In contrast, the supratemporal fenestrae of larger gryposuchines are mediolaterally wider than long, as is the case in adult Gavialis. This suggests that UPRMP 3094 is from an immature animal. Likewise, the temporal foramen is circular and not a slender slit, as in mature South American gryposuchines or Gavialis.
Two aspects of the skull table, however, are inconsistent with ontogenetic variation as observed in modern Gavialis. First, the frontoparietal suture intersects the supratemporal fenestral margin in UPRMP 3094. This condition occurs in older Gavialis in which positive allometry in the size of the fenestra pushes its anterior limit past the suture, but not in individuals with anteroposteriorly elongate fenestrae or in individuals of approximately the same size as UPRMP 3094. Second, the shallow fossa anterior to the fenestra does not occur in other gavialoids at any ontogenetic stage.
(c) Historical biogeography
The San Sebastián Formation was deposited in a basin resulting from subsidence of the Puerto Rico Trench (Monroe 1980). It consists of clay and sandy clay, with some local conglomerates and sandstones, overlying Cretaceous through Eocene rocks. The formation was predominantly formed in shallow marine, deltaic and estuarine depositional environments.
Siquisiquesuchus and Piscogavialis were also found in coastal settings (Kraus 1998; Brochu & Rincon 2004). These could be washout from inland sources, but the multiple occurrences, along with the presence of at least one form (Aktiogavialis) on what was then an island (Iturralde-Vinent & MacPhee 1999), argue that these occurrences might reflect the actual environment in which they lived.
The provenance of other Miocene gryposuchines is less clear. Gryposuchus colombianus is derived from non-marine deposits of Colombia (Kay & Madden 1997), but Gryposuchus and Ikanogavialis have been reported from the Urumaco sequence of Venezuela, which includes both marine and non-marine units (Sill 1970; Linares 2004; Sánchez-Villagra & Aguilera 2006). It is unclear whether Urumaco gavialoids are from fluvial or shallow marine portions of the sequence. Other South American gavialoids are from freshwater units (e.g. Rusconi 1935; Gasparini 1968; Bocquetin & Buffetaut 1981; Souza-Filho & Bocquetin 1989). There are no truly marine crocodylians alive today—those found in seawater also occur in freshwater (Ross 1998)—but in the absence of evidence placing fossil forms in coastal or marginal marine deposits, it is prudent to assume that these were freshwater animals.
Post-Palaeogene fossils unambiguously closer to G. gangeticus than to Gryposuchinae derive from the southern Asian mainland (Lull 1944) or Java (Janensch 1911; Delfino & de Vos 2006). All are from freshwater deposits, and Java was sometimes connected to the Asian mainland during the Neogene and the Quaternary (Voris 2000; Hall 2001). But derived gavialoids have been found in Neogene coastal deposits of the Arabian Peninsula (Rauhe et al. 1999) and the Quaternary of the Solomon Islands (Molnar 1982). Relationships of these forms to either Gryposuchinae or Gavialis are unknown, but they suggest a more complicated biogeographic scenario for Gavialoidea than ecology of the single living species would indicate.
Eogavialis africanum is from Late Eocene–Oligocene deposits in the Fayum Depression of Egypt that also represent both marine and non-marine deposits (Bown & Kraus 1988; Gingerich 1992; Gagnon 1997). It has been sampled from multiple horizons throughout the sequence (Andrews 1906; Buffetaut 1982), but we do not know whether the museum specimens observed come from both shallow marine and fluvial deposits.
Uncertainty over provenance, combined with unresolved phylogenetic relationships among gryposuchines, makes it difficult to assess whether the last common ancestor of Gavialis and gryposuchines was a coastal animal. But if we resolve gryposuchine relationships according to stratigraphic occurrence, with Aktiogavialis and Siquisiquesuchus forming successive outgroups to remaining members of the group, and if we assume that Eogavialis was a coastal form, then the last common ancestor of gryposuchines and Gavialis is most parsimoniously presumed to have occurred in a coastal setting.
Whether the ancestors of Gryposuchinae are of African or Asian origin is ambiguous on current phylogenetic estimates (Brochu & Rincon 2004), but an African origin has been preferred historically (e.g. Langston 1965; Buffetaut 1982; Langston & Gasparini 1997) largely because the Atlantic would be a much smaller barrier to cross than the Pacific and warm equatorial Atlantic currents run from east to west. The results of this analysis do not resolve the phylogenetic ambiguity, but the fact that the oldest member of the group (Aktiogavialis) is from the Greater Antilles rather than a Pacific locality supports the trans-Atlantic scenario.
Strictly speaking, the gavialoid fossil record does not falsify the hypothesis that vicariance explains the distribution of Neogene gavialoids. The fossil record is incomplete. But South America was isolated from the rest of Gondwana by the end of the Mesozoic, and although crocodyliforms are prominent members of Cretaceous Gondwanan faunas (e.g. Gasparini 1996; Buckley et al. 1997, 2000; Gomani 1997; Larsson & Gado 2000; Krause 2003; Sereno et al. 2003; Turner 2004; Pol & Apesteguía 2005; Candeiro & Martinelli 2006; Salisbury et al. 2006; Zaher et al. 2006), nothing related to Gavialis has been reported. Range extensions of roughly 75 Myr in South America and 65 Myr in Africa would be required to accommodate vicariance.
A coastal dispersal route along the North Atlantic is also problematic. Thoracosaurs are found in marginal marine deposits on both sides of the North Atlantic, but these are distantly related to gryposuchines (figure 3). Long-snouted crocodylians from coastal North America and Europe after the Early Eocene are tomistomines unrelated to gryposuchines (e.g. Auffenberg 1954; Antunes 1961; Buffetaut et al. 1984; Erickson & Sawyer 1996; Brochu 2001).
Other secondarily aquatic tetrapods are thought to have dispersed between Africa and the Neotropics since the Eocene, including seals (Deméré et al. 2003; Fyler et al. 2005), manatees and dugongs (Domning 2001; Vianna et al. 2006), and crocodiles (Crocodylus; Brochu 2001). The same is true for several non-aquatic tetrapods, including monkeys (Schrago & Russo 2003), rodents (Poux et al. 2006), geckos (Carranza et al. 2000) and skinks (Carranza & Arnold 2003). Clear examples of low-latitude dispersal across the Pacific to South America of coastal or freshwater tetrapods are lacking.
The oral cavity of extant Gavialis is keratinized. This is thought to limit osmotic loss of water in hypersaline environments among living crocodylians, and its presence in freshwater crocodylians (including Gavialis) was thought to be the evidence that crocodylians passed through a marine phase early in their history (Taplin et al. 1985; Taplin & Grigg 1989). This suggests at least partial tolerance of saltwater. But like living alligatorids (which, with rare exception, avoid saltwater), Gavialis lacks the large salt-excreting glands found on the tongues of crocodylids (Taplin et al. 1982; Taplin & Grigg 1989; Taylor et al. 1995; Jackson et al. 1996; Leslie & Taplin 2001). Whether absence in Gavialis is plesiomorphic or a derived loss is unknown, but if Gavialis is secondarily limited to freshwater, lingual salt glands (which leave no trace on the skeleton) might be expected in saltwater predecessors.
We therefore have a clade of derived gavialoids, at least some found in former coastal areas, in areas geographically separated from the ranges of known relatives in Africa and Asia. The fossils expected with vicariance- or circum-Atlantic dispersal-based models have not been found, and the oral morphology of Gavialis is consistent with saltwater tolerance. Taken together, these observations lend support for earlier suggestions that Neotropical gavialoids are descended from ancestors that crossed the Atlantic (Buffetaut 1982; Langston & Gasparini 1997).
Ongoing fieldwork in Puerto Rico will hopefully reveal more complete remains of Aktiogavialis and help resolve its relationships with other gavialoids, but fieldwork in South America and Africa is needed to allow a more complete and robust picture of gavialoid history throughout the Cenozoic. Further phylogenetic work on all crocodylians, living and extinct, is necessary to allow the integration of biogeographic information from fossils with physiological information from their living descendents.
Acknowledgments
For assistance in the field, we are grateful to the students in the UPR-Mayagüez 2006 stratigraphy class. Claudia Johnson provided information on geochronology, and access to comparative specimens was provided by M. Norell, M. Kearney, P. Holroyd and R. Kraus. This work was supported by NSF DEB 0444133 and NSF DEB 0228648 to C.A.B.
Supplementary Material
Character state codings used in phylogenetic analysis. Character states are discussed in Brochu (1999, 2004, 2006). Codings for Piscogavialis from Delfino et al. (2005) with modifications from observations of the holotype
References
- Andrews C.W. British Museum (Natural History); London, UK: 1906. A descriptive catalogue of the tertiary vertebrata of the Fayûm, Egypt. [Google Scholar]
- Annandale N. Fauna of the Chilka lake: reptiles and batrachia. Mem. Ind. Mus. 1915;5:167–174. [Google Scholar]
- Antunes M.T. Tomistoma lusitanica, crocodilien du Miocène du Portugal. Revista da Faculdade de Cièncias de Lisboa (ser. 2) 1961;9:5–88. [Google Scholar]
- Auffenberg W. Additional specimens of Gavialosuchus americanus (Sellards) from a new locality in Florida. Q.J. Fl. Acad. Sci. 1954;17:185–209. [Google Scholar]
- Benton M.J, Clark J.M. Archosaur phylogeny and the relationships of the Crocodylia. In: Benton M.J, editor. The phylogeny and classification of the tetrapods. Amphibians, reptiles, birds. vol. 1. Clarendon Press; Oxford, UK: 1988. pp. 295–338. [Google Scholar]
- Bocquetin J.C, Buffetaut E. Hesperogavialis cruxenti n. gen., n. sp., nouveau gavialide (Crocodylia, Eusuchai) du Miocene Superieur (Huayquerien) d'Urumaco (Venezuela) Géobios. 1981;14:415–419. [Google Scholar]
- Bown T.M, Kraus M.J. Geology and paleoenvironment of the Oligocene Jebel Qatrani Formation and adjacent rocks, Fayum Depression, Egypt. US Geol. Survey Prof. Pap. 1988;1452:1–60. [Google Scholar]
- Brochu C.A. Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis. Syst. Biol. 1997;46:479–522. doi: 10.1093/sysbio/46.3.479. doi:10.2307/2413693 [DOI] [PubMed] [Google Scholar]
- Brochu C.A. Congruence between physiology, phylogenetics, and the fossil record on crocodylian historical biogeography. In: Grigg G, Seebacher F, Franklin C.E, editors. Crocodilian biology and evolution. Surrey Beatty and Sons; Sydney, Australia: 2001. pp. 9–28. [Google Scholar]
- Brochu C.A. Phylogenetic approaches toward crocodylian history. Annu. Rev. Earth Planet. Sci. 2003;31:357–397. doi:10.1146/annurev.earth.31.100901.141308 [Google Scholar]
- Brochu C.A. A new gavialoid crocodylian from the Late Cretaceous of eastern North America and the phylogenetic relationships of thoracosaurs. J. Vert. Paleontol. 2004;24:610–633. doi:10.1671/0272-4634(2004)024[0610:ANLCGC]2.0.CO;2 [Google Scholar]
- Brochu C.A. Osteology and phylogenetic significance of Eosuchus minor (Marsh 1870), new combination, a longirostrine crocodylian from the Late Paleocene of North America. J. Paleontol. 2006;80:162–186. doi:10.1666/0022-3360(2006)080[0162:OAPSOE]2.0.CO;2 [Google Scholar]
- Brochu C.A, Rincon A.D. A gavialoid crocodylian from the Lower Miocene of Venezuela. Spec. Pap. Palaeontol. 2004;71:61–78. [Google Scholar]
- Brochu C.A, Nieves-Rivera A, Vélez-Juarbe J, Daza-Vaca J.D, Santos H. Tertiary crocodylians from Puerto Rico: evidence for Late Tertiary endemic crocodylians in the West Indies? Geobios. 2007;40:51–59. doi:10.1016/j.geobios.2005.10.008 [Google Scholar]
- Buckley G.A, Brochu C.A, Krause D.W. Hyperdiversity and the paleobiogeographic origins of the Late Cretaceous crocodyliforms of Madagascar. J. Vert. Paleontol. 1997;17:34A. [Google Scholar]
- Buckley G.A, Brochu C.A, Krause D.W, Pol D. A pug-nosed crocodyliform from the Late Cretaceous of Madagascar. Nature. 2000;405:941–944. doi: 10.1038/35016061. doi:10.1038/35016061 [DOI] [PubMed] [Google Scholar]
- Buffetaut E. Systematique, origine et évolution des Gavialidae Sud-Américains. Geobios, Memoire Special. 1982;6:127–140. [Google Scholar]
- Buffetaut E, Crouzel F, Juillard F, Stigliani F. Le crocodilien longirostre Gavialosuchus dans le Miocène moyen de Polastron (Gers, France) Geobios. 1984;17:113–117. doi:10.1016/S0016-6995(84)80009-1 [Google Scholar]
- Candeiro C.R.A, Martinelli A.G. A review of paleogeographical and chronostratigraphical distribution of mesoeucrocodylian species from the upper Cretaceous beds from the Bauru (Brazil) and Neuquén (Argentina) groups, southern South America. J. South Am. Earth Sci. 2006;22:116–112. doi:10.1016/j.jsames.2006.08.001 [Google Scholar]
- Carranza S, Arnold E.N. Investigating the origin of transoceanic distributions: mtDNA shows Mabuya lizards (Reptilia: Scincidae) crossed the Atlantic twice. Syst. Biodiv. 2003;1:275–282. doi:10.1017/S1477200003001099 [Google Scholar]
- Carranza S, Arnold E.N, Mateo J.A, Lopez-Jurado L.F. Long-distance colonization and radiation in gekkonid lizards, Tarentola (Reptilia: Gekkonidae), revealed by mitochondrial DNA sequences. Proc. R. Soc. B. 2000;267:637–649. doi: 10.1098/rspb.2000.1050. doi:10.1098/rspb.2000.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino M, de Vos J. A revision of Dubois crocodylians: Gavialis bengawanicus and Crocodylus ossifragus from the Early Pleistocene Homo beds of Java. J. Vert. Paleontol. 2006;26:55A. [Google Scholar]
- Delfino M, Piras P, Smith T. Anatomy and phylogeny of the gavialoid Eosuchus lerichei from the Paleocene of Europe. Acta Palaeontologica Polonica. 2005;50:565–580. [Google Scholar]
- Deméré T.A, Berta A, Adam P.J. Pinnipedimorph evolutionary biogeography. Bull. Am. Mus. Nat. Hist. 2003;279:32–76. [Google Scholar]
- Densmore L.D. Biochemical and immunological systematics of the order Crocodilia. In: Hecht M.K, Wallace B, Prance G.H, editors. Evolutionary biology. vol. 16. Plenum Press; New York, NY: 1983. pp. 397–465. [Google Scholar]
- Densmore L.D, Owen R.D. Molecular systematics of the order Crocodilia. Am. Zool. 1989;29:831–841. [Google Scholar]
- Domning D.P. Evolution of the Sirenia and Desmostylia. In: Mazin J.-M, Buffrenil V, editors. Secondary adaptation of tetrapods to life in water. Verlag Dr. Friedrich Pfeil; Munich, Germany: 2001. pp. 151–168. [Google Scholar]
- Erickson B.R. Crocodilians of the Black Mingo Group (Paleocene) of the South Carolina Coastal Plain. Trans. Am. Phil. Soc. 1998;88:196–214. doi:10.2307/1006674 [Google Scholar]
- Erickson B.R, Sawyer G.T. The estuarine crocodile Gavialosuchus carolinensis n. sp. (Crocodylia: Eusuchia) from the Late Oligocene of South Carolina, North America. Monogr. Sci. Mus. Minn. (Paleontology) 1996;3:1–47. [Google Scholar]
- Frost S.H, Harbour J.L, Beach D.K, Realini M.J, Harris P.M. Oligocene reef tract development, southwestern Puerto Rico. Sedimenta. 1983;9:1–144. [Google Scholar]
- Fyler C.A, Reeder T.W, Berta A, Antonelis G, Aguilar A, Androukaki E. Historical biogeography and phylogeny of monachine seals (Pinnipedia: Phocidae) based on mitochondrial and nuclear DNA data. J. Biogeogr. 2005;32:1267–1279. doi:10.1111/j.1365-2699.2005.01281.x [Google Scholar]
- Gagnon M. Ecological diversity and community ecology in the Fayum sequence (Egypt) J. Human Evol. 1997;32:133–160. doi: 10.1006/jhev.1996.0107. doi:10.1006/jhev.1996.0107 [DOI] [PubMed] [Google Scholar]
- Gallagher W.B, Parris D.C, Spamer E.E. Paleontology, biostratigraphy, and depositional environments of the Cretaceous–Tertiary transition in the New Jersey Coastal Plain. The Mosasaur. 1986;3:1–35. [Google Scholar]
- Gasparini Z. Nuevas restos de Rhamphostomopsis neogaeus (Burm.) Rusconi 1933 (Reptilia, Crocodilia) del “Mesopotamiense” (Plioceno medio-superior) de Argentina. Ameghiniana. 1968;5:299–311. [Google Scholar]
- Gasparini Z. Biogeographic evolution of the South American crocodilians. Münchner Geowissenschaftliche Abhandlungen. 1996;30:159–184. [Google Scholar]
- Gatesy J, Amato G, Norell M, DeSalle R, Hayashi C. Combined support for wholesale taxic atavism in gavialine crocodylians. Syst. Biol. 2003;52:403–422. doi: 10.1080/10635150390197037. doi:10.1080/10635150390197037 [DOI] [PubMed] [Google Scholar]
- Gingerich P.D. Marine mammals (Cetacea and Sirenia) from the Eocene of Gebel Mokattam and Fayum, Egypt: stratigraphy, age, and paleoenvironments. Univ. Mich. Pap. Paleontol. 1992;30:1–84. [Google Scholar]
- Gomani E.M. A crocodyliform from the Early Cretaceous Dinosaur Beds, northern Malawi. J. Vert. Paleontol. 1997;17:280–294. [Google Scholar]
- Gürich G. Gryposuchus jessei, ein neues schmalsnauziges Krokodil aus den jüngeren Ablagerungen des oberen Amazonas-Gebietes. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten. 1912;29:59–71. [Google Scholar]
- Hall R. Cenozoic reconstructions of SE Asia and the SW Pacific: changing patterns of land and sea. In: Metcalfe I, Smith J.M.B, Morwood M, Davidson I.D, editors. Faunal and floral migrations and evolution in SE Asia–Australia. A.A. Balkema; Lisse, The Netherlands: 2001. pp. 35–56. [Google Scholar]
- Harshman J, Huddleston C.J, Bollback J.P, Parsons T.J, Braun M.J. True and false gharials: a nuclear gene phylogeny of Crocodylia. Syst. Biol. 2003;52:386–402. doi: 10.1080/10635150390197028. doi:10.1080/10635150390197028 [DOI] [PubMed] [Google Scholar]
- Hass C.A, Hoffman M.A, Densmore L.D, Maxson L.R. Crocodilian evolution: insights from immunological data. Mol. Phylogenet. Evol. 1992;1:193–201. doi: 10.1016/1055-7903(92)90015-9. doi:10.1016/1055-7903(92)90015-9 [DOI] [PubMed] [Google Scholar]
- Hua S, Jouve S. A primitive gavialoid from the Paleocene of Morocco. J. Vert. Paleontol. 2004;24:341–350. doi:10.1671/1104 [Google Scholar]
- Iturralde-Vinent M.A, MacPhee R.D.E. Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bull. Am. Mus. Nat. Hist. 1999;238:1–95. [Google Scholar]
- Jackson K, Butler D.G, Brooks D.R. Habitat and phylogeny influence salinity discrimination in crocodilians: implications for osmoregulatory physiology and historical biogeography. Biol. J. Linn. Soc. 1996;58:371–383. doi:10.1006/bijl.1996.0042 [Google Scholar]
- Janensch W. Die Reptilienreste (exkl. Schildkröten) In: Selenka M.L, Blanckenhorn M, editors. Die Pithecanthropus-Schichten auf Java. Wilhelm Engelmann; Leipzig, Germany: 1911. pp. 61–74. [Google Scholar]
- Janke A, Gullberg A, Hughes S, Aggarwal R.K, Arnason U. Mitogenomic analyses place the gharial (Gavialis gangeticus) on the crocodile tree and provide pre-K/T divergence times for most crocodilians. J. Mol. Evol. 2005;61:620–626. doi: 10.1007/s00239-004-0336-9. doi:10.1007/s00239-004-0336-9 [DOI] [PubMed] [Google Scholar]
- Johnson C.C, Ramirez W.R, Leckie R.M, Hernandez S.Y, Barrow E.A, Hegewald M, Velez J. Oligocene reef deposits linked to ODP site 999 with strontium isotope stratigraphy. Geol. Soc. Am. Abstr. Prog. 2006;38:557. [Google Scholar]
- Jouve S, Iarochene M, Bouya B, Amaghzaz M. New material of Argochampsa krebsi (Crocodylia: Gavialoidea) from the Lower Paleocene of the Oulad Abdoun Basin (Morocco): phylogenetic implications. Geobios. 2006;39:817–832. doi:10.1016/j.geobios.2005.07.003 [Google Scholar]
- Kälin J.A. Beiträge zur vergleichenden Osteologie des Crocodilidenschädels. Zoologische Jahrbücher. 1933;57:535–714. [Google Scholar]
- Kay R.F, Madden R.H. Paleogeography and paleoecology. In: Kay R.F, Madden R.H, Cifelli R.L, Flynn J.J, editors. Vertebrate paleontology in the neotropics: the Miocene fauna of La Venta, Colombia. Smithsonian Institution Press; Washington, DC: 1997. pp. 520–550. [Google Scholar]
- Köken E. Thoracosaurus macrorhynchus Bl. aus der Tuffkreide von Maastricht. Abdruck a.d. Zeitschrift der Deutschen geologische Gesselschaft. 1888;1888:754–773. [Google Scholar]
- Kraus R. The cranium of Piscogavialis jugaliperforatus n. gen., n. sp. (Gavialidae, Crocodylia) from the Miocene of Peru. Paläontologische Zeitschrift. 1998;72:389–406. [Google Scholar]
- Krause D.W. Late Cretaceous vertebrates of Madagascar: a window into Gondwanan biogeography at the end of the age of dinosaurs. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago Press; Chicago, IL: 2003. pp. 40–47. [Google Scholar]
- Langston W. Fossil crocodilians from Colombia and the Cenozoic history of the Crocodilia in South America. Univ. Calif. Publ. Geol. Sci. 1965;52:1–152. [Google Scholar]
- Langston W. The crocodilian skull in historical perspective. In: Gans C, Parsons T, editors. Biology of the reptilia. Morphology D. vol. 4. Academic Press; London, UK: 1973. pp. 263–284. [Google Scholar]
- Langston W, Gasparini Z. Crocodilians, Gryposuchus, and the South American gavials. In: Kay R.F, Madden R.H, Cifelli R.L, Flynn J.J, editors. Vertebrate paleontology in the neotropics: the Miocene fauna of La Venta, Colombia. Smithsonian Institution; Washington, DC: 1997. pp. 113–154. [Google Scholar]
- Larsson H.C.E, Gado B. A new Early Cretaceous crocodyliform from Niger. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen. 2000;217:131–141. [Google Scholar]
- Laurent Y, Buffetaut E, Le Loeff J. Un crane de thoracosaurine (Crocodylia, Crocodylidae) dans le Maastrichtien Superieur du sud de la France. Oryctos. 2000;3:19–27. [Google Scholar]
- Leslie A.J, Taplin L.E. Recent developments in osmoregulation of crocodilians. In: Grigg G, Seebacher F, Franklin C.E, editors. Crocodilian biology and evolution. Surrey Beatty and Sons; Chipping Norton, UK; Sydney, Australia: 2001. pp. 265–279. [Google Scholar]
- Linares O.J. Bioestratigrafia de la fauna de mamiferos de las Formaciones Socorro, Urumaco y Codore (Mioceno Medio–Plioceno Temprano) de la region de Urumaco, Falcon, Venezuela. Paleobiologia Neotropical. 2004;1:1–26. [Google Scholar]
- Lull R.S. Fossil gavials from India. Am. J. Sci. 1944;242:417–430. [Google Scholar]
- MacPhee R.D.E, Wyss A.R. Oligo–Miocene vertebrates from Puerto Rico, with a catalog of localities. Am. Mus. Novit. 1990;2965:1–45. [Google Scholar]
- McAliley L.R, Willis R.E, Ray D.A, White P.S, Brochu C.A, Densmore L.D. Are crocodiles really monophyletic?—evidence for subdivisions from sequence and morphological data. Mol. Phylogenet. Evol. 2006;39:16–32. doi: 10.1016/j.ympev.2006.01.012. doi:10.1016/j.ympev.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Molnar R.E. A longirostrine crocodilian from Murua (Woodlark), Solomon Sea. Mem. Queensl. Mus. 1982;20:675–685. [Google Scholar]
- Monroe W.H. Geology of the middle Tertiary formations of Puerto Rico. US Geol. Survey Prof. Pap. 1980;953:1–93. [Google Scholar]
- Norell M.A. The higher level relationships of the extant Crocodylia. J. Herpetol. 1989;23:325–335. doi:10.2307/1564042 [Google Scholar]
- Piveteau J. Études sur quelques amphibiens et reptiles fossiles, II: Reptile du Montien. Annales de Paléontologie. 1927;16:29–37. [Google Scholar]
- Pol D, Apesteguía S. New Araripesuchus remains from the Early Late Cretaceous (Cenomanian–Turonian) of Patagonia. Am. Mus. Novit. 2005;3490:1–38. doi:10.1206/0003-0082(2005)490[0001:NARFTE]2.0.CO;2 [Google Scholar]
- Poux C, Chevret P, Huchon D, de Jong W.W, Douzery E.J.P. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Syst. Biol. 2006;55:228–244. doi: 10.1080/10635150500481390. doi:10.1080/10635150500481390 [DOI] [PubMed] [Google Scholar]
- Ramirez W.R, Johnson C.C, Martinez M, Torres M.C, Ortiz V. Strontium isotope stratigraphy from Kuphus incrassatus, Cretaceous limestones, Puerto Rico. Geol. Soc. Am. Abstr. Prog. 2006;38:90. [Google Scholar]
- Rauhe M, Frey E, Pemberton D.S, Rossmann T. Fossil crocodilians from the Late Miocene Baynunah Formation of the Emirate of Abu Dhabi, United Arab Emirates: osteology and palaeoecology. In: Whybrow P.J, Hill A, editors. Fossil vertebrates of Arabia. Yale University Press; New Haven, CT: 1999. pp. 163–185. [Google Scholar]
- Robb A.J. Vertebrate fossils from the Upper Cretaceous (Merchantville Formation: Early Campanian) Graham Brickyard Locality of New Jersey. The Mosasaur. 2004;7:75–88. [Google Scholar]
- Ross J.P. International Union for the Conservation of Nature; Gland, Switzerland: 1998. Crocodiles—status survey and conservation action plan. [Google Scholar]
- Rusconi C. Observacones sobre los gaviales fosiles Argentinos. Anales de la S.C. Argentina. 1935;119:203–214. [Google Scholar]
- Salisbury S.W, Willis P.M.A. A new crocodylian from the Early Eocene of southeastern Queensland and a preliminary investigation of the phylogenetic relationships of crocodyloids. Alcheringa. 1996;20:179–227. [Google Scholar]
- Salisbury S.W, Molnar R.E, Frey E, Willis P.M.A. The origin of modern crocodyliforms: new evidence from the Cretaceous of Australia. Proc. R. Soc. B. 2006;273:2439–2448. doi: 10.1098/rspb.2006.3613. doi:10.1098/rspb.2006.3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villagra M.R, Aguilera O.A. Neogene vertebrates from Urumaco, Falcón State, Venezuela: diversity and significance. J. Syst. Palaeontol. 2006;4:213–220. doi:10.1017/S1477201906001829 [Google Scholar]
- Schrago C.G, Russo C.A.M. Timing the origin of New World monkeys. Mol. Biol. Evol. 2003;20:1620–1625. doi: 10.1093/molbev/msg172. doi:10.1093/molbev/msg172 [DOI] [PubMed] [Google Scholar]
- Schwimmer D.R. Late Cretaceous fossils from the Blufftown Formation (Campanian) in western Georgia. The Mosasaur. 1986;3:109–123. [Google Scholar]
- Seiglie G.A, Moussa M.T. Late Oligocene–Pliocene transgressive–regressive cycles of sedimentation in northwestern Puerto Rico. Am. Assoc. Petrol. Geol. Mem. 1984;36:89–95. [Google Scholar]
- Sereno P.C, Sidor C.A, Larsson H.C.E, Gado B. A new notosuchian from the Early Cretaceous of Niger. J. Vert. Paleontol. 2003;23:477–482. doi:10.1671/0272-4634(2003)023[0477:ANNFTE]2.0.CO;2 [Google Scholar]
- Sill W.D. Nota preliminar sobre un nuevo gavial del Plioceno de Venezuela y una discusion de los gaviales sudamericanos. Ameghiniana. 1970;7:151–159. [Google Scholar]
- Singh L.A.K. Distribution of Gavialis gangeticus. Hamadryad. 1991;16:39–46. [Google Scholar]
- Smith M.A. Loricata, Testudines. vol. 1. Taylor and Francis; London, UK: 1933. The reptiles of British India, including Ceylon and Burma: reptilia and amphibia. [Google Scholar]
- Souza-Filho J.P, Bocquetin J.C. Anais do XI Congresso Brasiliero de Paleontologia. 1989. Brasilosuchus mendesi, n.g., sp. nov., um novo representante da familia Gavialidae no Neogene do Acre, Brasil. pp. 457–463. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP*. Phylogenetic analysis using parsimony (*and Other Methods), version 4.0b10. [Google Scholar]
- Taplin L.E, Grigg G.C. Historical zoogeography of the Eusuchian crocodilians: a physiological perspective. Am. Zool. 1989;29:885–901. [Google Scholar]
- Taplin L.E, Grigg G.C, Harlow P, Ellis T.M, Dunson W.A. Lingual salt glands in Crocodylus acutus and C. johnstoni, and their absence from Alligator mississippiensis and Caiman crocodilus. J. Comp. Physiol. 1982;149:43–47. [Google Scholar]
- Taplin L.E, Grigg G.C, Beard L. Salt gland function in fresh water crocodiles: evidence for a marine phase in Eusuchian evolution? In: Grigg G, Shine R, Ehmann H, editors. Biology of Australasian frogs and reptiles. Surrey Beatty and Sons; Sydney, Australia: 1985. pp. 403–410. [Google Scholar]
- Taylor G.C, Franklin C.E, Grigg G.C. Salt loading stimulates secretion by the lingual salt glands in unrestrained Crocodylus porosus. J. Exp. Zool. 1995;272:490–495. doi:10.1002/jez.1402720611 [Google Scholar]
- Troedsson G.T. On crocodilian remains from the Danian of Sweden. Lunds Universitets Årsskrift N.F. 1924;20:1–75. [Google Scholar]
- Turner A.H. Crocodyliform biogeography during the Cretaceous: evidence of Gondwanan vicariance from biogeographical analysis. Proc. R. Soc. B. 2004;271:2003–2009. doi: 10.1098/rspb.2004.2840. doi:10.1098/rspb.2004.2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bold W.A. Middle Tertiary Ostracoda from northwestern Puerto Rico. Micropaleontology. 1965;11:381–414. doi:10.2307/1484777 [Google Scholar]
- Vianna J.A, et al. Phylogeography, phylogeny and hybridization in trichechid sirenians: implications for manatee conservation. Mol. Ecol. 2006;15:433–447. doi: 10.1111/j.1365-294X.2005.02771.x. doi:10.1111/j.1365-294X.2005.02771.x [DOI] [PubMed] [Google Scholar]
- Voris H.K. Maps of Pleistocene sea levels in southeast Asia: shorelines, river systems and time durations. J. Biogeogr. 2000;27:1153–1167. doi:10.1046/j.1365-2699.2000.00489.x [Google Scholar]
- Ward W.C, Scharlach R.A, Hartley J.R. Geology of the North Coast ground-water Province of Puerto Rico. In: Renken R.A, Ward W.C, Gill I.P, Gomez-Gomez F, Rodriguez-Martinez J, editors. Geology and hydrogeology of the Caribbean Islands aquifer system of the Commonwealth of Puerto Rico and the U.S. Virgin Islands. U.S. Geological Survey Professional Paper 1419. 2002. pp. 45–76. [Google Scholar]
- Weems R.E. Reptile remains from the Fisher/Sullivan site. Vir. Div. Miner. Resour. Publ. 1999;152:101–121. [Google Scholar]
- Whitaker R, Basu D. The gharial (Gavialis gangeticus): a review. J. Bombay Nat. Hist. Soc. 1983;79:531–548. [Google Scholar]
- White P.S, Densmore L.D. DNA sequence alignments and data analysis methods: their effect on the recovery of crocodylian relationships. In: Grigg G, Seebacher F, Franklin C.E, editors. Crocodilian biology and evolution. Surrey Beatty and Sons; Sydney, Australia: 2001. pp. 29–37. [Google Scholar]
- Zaher H, Pol D, Carvalho A.B, Riccomini C, Campos D.A, Nava W. Redescription of the cranial morphology of Mariliasuchus amarali, and its phylogenetic affinities (Crocodyliformes, Notosuchia) Am. Mus. Novit. 2006;3512:1–40. doi:10.1206/0003-0082(2006)3512[1:ROTCMO]2.0.CO;2 [Google Scholar]
- Zarski M, Jakubowski G, Gawor-Biedowa E. The first Polish find of Lower Paleocene crocodile Thoracosaurus Leidy, 1852: geological and palaeontological description. Geol. Q. 1998;42:141–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Character state codings used in phylogenetic analysis. Character states are discussed in Brochu (1999, 2004, 2006). Codings for Piscogavialis from Delfino et al. (2005) with modifications from observations of the holotype