Abstract
Low-level radioactive contamination may affect choice of breeding site and life-history decisions if (i) radioactivity directly affects body condition or (ii) it affects resource abundance that then secondarily influences reproductive decisions. We tested the effects of radioactive contamination on nest-site choice and reproduction in a community of hole nesting birds by putting up nest boxes in areas differing in levels of background radiation. Great tit Parus major and pied flycatcher Ficedula hypoleuca significantly avoided nest boxes in heavily contaminated areas, with a stronger effect in flycatchers than in tits. These preferences could not be attributed to variation in habitat quality or resource abundance, as determined by analyses of habitat use and the relationship between radiation and life-history characters. Likewise, none of these effects could be attributed to individuals of a specific age breeding in the most contaminated areas. Laying date and clutch size were not significantly related to dose rate in either species. Hatching success was depressed by elevated radioactive contamination, interacting with habitat in the great tit and with laying date in the pied flycatcher. Interspecific differences in effects of radiation on nest-site choice suggest that species respond in a species-specific manner to radiation, perhaps related to differences in migratory habits. We suggest that individual body condition rather than secondary effects of radiation on resource abundance account for the effects on nest box use and hatching success.
Keywords: clutch size, dose rate, hatching success, laying date
1. Introduction
Radioactivity occurs naturally or as a consequence of release of radioactive material by humans into the environment. The effects of low-level radiation on plants and animals, including humans, have attracted considerable attention by scientists since the Hiroshima and Nagasaki nuclear bombs in 1945. However, the final report on mutations in children of atomic bomb survivors showed little or no effect (Neel et al. 1988). Likewise, a recent report on the biological consequences of the accident at the Chernobyl nuclear power plant in 1986 has suggested effects on excess deaths in humans that are well below those predicted after the accident (Chernobyl Forum 2005). However, the main conclusion remaining after 20 years of research of the biological consequences of the Chernobyl disaster is that our current state of knowledge is extremely limited, with little or no information on ecological or evolutionary consequences (Mousseau et al. 2005; Møller & Mousseau 2006).
The Chernobyl accident contaminated more than 5000 km2 in Ukraine and Belarus to an extent that these areas have been completely depopulated (Anonymous 1996). Immediately following the explosion at the Chernobyl reactor, the main sources of radiation were 131I, 137Cs and 90Sr (Shestopalov 1996). Owing to its short half-life (8 days), radioactive 131I is no longer a problem. However, both 137Cs and 90Sr are strong radiation emitters with half-lives of 30 and 29 years, respectively (Shestopalov 1996), and they will thus be present in significant amounts for many hundred years to come. Vast areas outside the exclusion zone are affected by lower levels of radiation that greatly exceed natural background levels and such contamination may have consequences for survival and reproduction of humans and other organisms. Surprisingly, there have been only few and scattered efforts to quantify the effects of contamination on ecological phenomena such as the abundance and distribution of plants and animals.
Likewise, no study has so far investigated how plants and animals under field conditions respond to variation in low-level radiation when selecting a site for reproduction (Møller & Mousseau 2006). If such phenotypic responses to levels of background radiation could be demonstrated, this would raise questions about potential mechanisms. We propose three different mechanisms for such a response. First, individuals prospecting for a future site of reproduction might use the presence of conspecifics or their reproductive success as a cue to suitable sites (Boulinier & Danchin 1997; Danchin & Wagner 1997). If low-level radiation has negative effects on reproductive success, as shown in at least one study (Møller et al. 2005a), we would expect lower levels of recruitment to such sites. Second, if low-level radiation has negative effects on the abundance of resources, avoidance of contaminated sites would simply reflect an ideal free distribution of individuals in relation to food abundance (Fretwell & Lucas 1970). Third, individuals living in contaminated areas may suffer physiological consequences in terms of reduced levels of antioxidants (Chaialo et al. 1991; Bazhan 1998; Ben-Amotz et al. 1998; Ivaniota et al. 1998; Neyfakh et al. 1998a,b; Lykholat & Chernaya 1999; Kumerova et al. 2000; Møller et al. 2005b), and such reductions can have important consequences for reproductive decisions, including whether or not to reproduce, clutch size and investment in eggs (Møller et al. 2005a). Therefore, individuals with reduced levels of antioxidants may be less likely to settle in a given site than individuals with adequate levels, creating a correlation between condition and background dose rate.
The objective of this study was to test if free-living animals choose sites for reproduction related to the local level of background radiation. To this end, we investigated nest box choice by small passerine birds in an area near Chernobyl with a very large spatial heterogeneity in background radiation levels. Therefore, we predicted a negative relationship between dose rates at nest boxes and probability of occupation, if radiation level affected nest box choice. A secondary objective of the study was to test the potential mechanisms accounting for an association between nest box use and radiation. More specifically, we predicted that the previous presence of conspecifics would be an unlikely mechanism in areas where boxes have never previously been supplied, thus expecting an effect of year, with conspecific presence being more important in the second and the third year when compared with the first year. If resource abundance were a determining factor, we would expect measures of reproductive success to be negatively related to radiation level, because reduced food availability would cause partial reproductive failure.
2. Material and methods
(a) Study sites
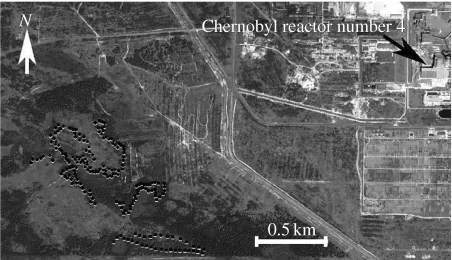
We investigated nest box use and reproductive parameters in a 250 ha area in the Red Forest, Chernobyl, where levels of radioactive contamination reach a level that is greater than 2000 times the natural background level. In 2002–2003, we erected 232 nest boxes of a standard size (20 cm×20 cm×40 cm) with a 32 mm diameter hole. All boxes were placed at a distance of at least 50 m between boxes at a height of 1.5–2.0 m. High levels of spatial heterogeneity in radiation levels (Shestopalov 1996; figure 1) provided an ideal setting for investigating nest box choice and reproduction.
Figure 1.
Location of nest boxes in the Chernobyl site with radiation levels indicated for each box.
The habitat was relatively open forest with a mixture of deciduous and coniferous trees and shrubs. The oldest trees were less than 100 years old. S. Gaschak classified the habitat surrounding all nest boxes with respect to four different axes, and the involvement of a single person assured that there was no inter-observer variability: (i) tree species (birch Betula sp.; birch with aspen Populus sp. and alder Alnus sp.; pine Pinus sp.), (ii) age of stands (young trees without natural holes; older trees with natural holes), (iii) undergrowth (absent; sparse; dense)), and (iv) habitat humidity (dry; humid; wet with open water).
(b) Recording occupancy and nest box contents
S. Gaschak visited nest boxes during April–May 2003–2005 to check for occupancy. He identified species from the adults, eggs and nests present. He estimated laying date from the presence of clutches that had not been completed, assuming that one egg was laid per day (Cramp & Perrins 1988–1993). Likewise, he used the age of nestlings to estimate date of laying of the first egg, assuming an incubation period of 13.9 and 14.75 days, respectively, for great tits Parus major and pied flycatchers Ficedula hypoleuca, and a nestling period of 18.9 and 14.6 days, respectively, for the same species (Cramp & Perrins 1988–1993).
He recorded clutch size as the number of eggs in complete clutches and brood size as the number of young in the nest after hatching and before fledging, respectively.
Hatching success was estimated as brood size at hatching divided by clutch size, and fledging success brood size at fledging divided by brood size at hatching.
In May–June 2006, we captured 36 adult great tits and pied flycatchers at nest boxes and determined their age using patterns of moult of wing coverts to distinguish yearlings from older individuals (Svensson 1984).
(c) Recording background radiation levels at nest boxes
As an estimate of background radiation levels, we relied on a dataset of air-gamma-survey of the Chernobyl zone. This dataset includes geo-positioned data of 137Cs and 90Sr deposition in a grid of 100 m×100 m as of 1 January 1992. Using corresponding software (MapInfo Prof.), we sampled from the database all data that were within a radius of 500 m from each nest box. Figure 1 shows the position of nest boxes and their radiation level. The choice of a radius of 500 m was based on the assumption that most birds remained within an area with a radius of 500 m. This assumption is supported by the size of the home range of tits and flycatchers during breeding (Perrins 1979; Lundberg & Alatalo 1992). We cross-validated these data by using our own field measurements of background radiation at the ground level using a hand-held Inspector (model: Inspector, SE International, Inc., Summertown, TN, USA) dosimeter at sample of 28 nest boxes during May–June 2006. We found a strong positive relationship between these two series of measurements (F=210.99, d.f.=1, 26, r2=0.89, p<0.0001, slope (s.e.)=1.15 (0.08)).
(d) Statistical analyses
We used partial Mantel tests (Smouse et al. 1986) to investigate relationships between dose rate, nest box occupation and reproductive variables, using the software XLStat 2006 with 1000 permutations in each test (Addinsoft 2006). Hatching success, fledging success and breeding success were square-root arcsine-transformed, while dose rate was log-transformed to meet requirements for normally distributed data (none of the variables differed significantly from normal distributions after transformation).
We modelled occupation rate (in great tit never occupied, occupied once or occupied twice; in pied flycatcher never occupied or occupied once) in relation to log-transformed dose rate of boxes, using logistic regression with a logit link function. These models included year as a factor in order to control for differences in abundance among years, and year by dose rate interactions in order to test for differences in effects of dose rate among years.
We modelled laying date, clutch size, hatching success and fledging success by using year, dose rate, habitat variables and their two-way interactions as predictor variables, using Akaike's information criterion to develop best-fit models for these three reproductive variables (Burnham & Anderson 2002). Habitat variables were used as a factor. In addition, because clutch size may depend on laying date, we included laying date and its interaction with dose rate in the analyses. For hatching and fledging success, we included laying date and clutch size as additional variables.
We determined whether age of breeding birds was associated with level of background radiation at nest boxes by assessing whether log-transformed radiation level differed between yearlings and older individuals, and whether this effect differed between great tits and pied flycatchers by testing for an interaction between age and species.
Data were missing for some variables due to complete nest failure, causing sample sizes to differ among tests. Variables reported are means (s.e.).
3. Results
(a) Choice of breeding site and rate of radiation
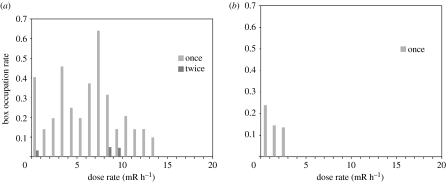
Dose rates for nest boxes ranged from 0.54 to 16.7 mR h−1, with a mean (s.e.) of 6.25 (0.30), N=231. For pied flycatchers, but not for great tits, we found a significantly decreasing relationship between occupation rate and dose rate (figure 2; table 1). There was also a significant year effect for tits, but not for pied flycatchers (table 1, models 1 and 3). Interactions between year and dose rate were non-significant. Partial Mantel tests for occupation in relation to dose rate after controlling for distance were statistically significant for both species (great tit: r=−0.268, p<0.0001; pied flycatcher: r=−0.396, p<0.0001). Therefore, occupation rate was negatively related to dose rate after adjusting for distance.
Figure 2.
Nest box choice (proportion of boxes occupied) in relation to dose rate (mR h−1) at nest boxes for (a) great tits Parus major and (b) pied flycatchers Ficedula hypoleuca. The number of boxes for the 20 radiation intervals were 59, 7, 15, 13, 8, 10, 8, 14, 19, 21, 19, 7, 14, 10, 2, 3, 1, 0, 0 and 0, respectively.
Table 1.
Nest box use by great tits and pied flycatchers in relation to year and dose rate (models 1 and 3) and dose rate and habitat (models 2 and 4). (The statistics of the models were for model 1: χ62=26.62, p<0.0001, model 2: χ62=14.01, p=0.030, model 3: χ32=38.20, p<0.0001 and model 4: χ12=34.32, p<0.0001.)
| variable | d.f. | wald | p | slope (s.e.) |
|---|---|---|---|---|
| tits | ||||
| model 1 | ||||
| year | 4 | 13.95 | 0.0074 | |
| log (dose rate) | 2 | 4.24 | 0.12 | −0.70 (1.04) |
| model 2 | ||||
| age of stand | 2 | 3.11 | 0.21 | |
| log (dose rate) | 2 | 9.85 | 0.073 | −1.49 (0.64) |
| log (dose rate)×age of stand | 2 | 5.13 | 0.037 | |
| pied flycatcher | ||||
| model 3 | ||||
| year | 2 | 3.57 | 0.17 | |
| log (dose rate) | 1 | 14.82 | <0.0001 | −3.59 (0.93) |
| model 4 | ||||
| log (dose rate) | 1 | 16.03 | <0.0001 | −3.78 (0.94) |
We checked the robustness of this conclusion by entering habitat variables as additional predictors in the models. For great tits, there was a significant interaction between age of stands and dose rate (table 1, model 2). We also entered age of stand in the partial Mantel test for great tits, but this did not affect the conclusions. For pied flycatchers, only dose rate was entered as a significant predictor (table 1, model 4). The effect of dose rate differed significantly between the two species, with the effect being stronger in flycatchers than in great tits (F=22.79, d.f.=1, p<0.0001). Therefore, boxes with pied flycatchers had significantly lower dose rates than boxes with great tits (pied flycatcher: 1.03 (0.18), N=16; great tit: 4.88 (0.47), N=71; t=11.85, d.f.=85, p<0.0001).
There was no difference in level of radiation between age classes (F=0.07, d.f.=1, 34, p=0.79; mean (s.e.) for yearlings: 0.04 (0.33), N=24; adults: 0.16 (0.16), N=12). Furthermore, there was no significant interaction between age and species (F=0.13, d.f.=1, 32, p=0.88), while the effect of species was significant, as already shown above (F=4.57, d.f.=1, 32, p=0.019). There was no age difference in sex of captured birds (33% males of 24 yearlings and 33% males of 12 older birds).
(b) Laying date, clutch size and reproductive success and rate of radiation
Summary statistics for reproductive variables are reported in table 2. Laying date was related to undergrowth as the only significant predictor (table 3).
Table 2.
Summary statistics for reproductive variables of great tits and pied flycatchers in Chernobyl. (Values are means (s.e.))
| great tit | pied flycatcher | |
|---|---|---|
| laying date | 2 May (1) | 19 May (2) |
| clutch size | 9.56 (0.29) | 5.94 (0.17) |
| hatching success (%) | 72.55 (4.23) | 83.39 (0.06) |
| fledging success (%) | 82.82 (4.20) | 97.42 (0.01) |
| N | 105 | 32 |
Table 3.
Models of reproductive variables of great tits and pied flycatchers in relation to dose rate, laying date, habitat, year and their two-way interactions. (The statistics for the models were F=8.64, d.f.=1, 81, r2=0.10, p=0.004, F=29.62, d.f.=2, 80, r2=0.43, p<0.0001, F=3.91, d.f.=9, 55, r2=0.39, p=0.0007, F=6.39, d.f.=2, 49, r2=0.21, p=0.0034 and F=4.40, d.f.=4, 47, r2=0.27, p=0.0042 for great tit, and F=4.25, d.f.=1, 28, r2=0.13, p=0.049, F=23.41, d.f.=2, 27, r2=0.63, p<0.0001, F=9.36, d.f.=4, 23, r2=0.62, p<0.0001 and F=4.82, d.f.=1, 20, r2=0.19, p=0.04 for pied flycatcher.)
| variable | d.f. | SS | F | p | slope (s.e.) |
|---|---|---|---|---|---|
| great tit | |||||
| laying date: | |||||
| undergrowth | 1 | 1089.61 | 8.65 | 0.0005 | −2.00 (0.56) |
| clutch size: | |||||
| date | 1 | 233.98 | 49.81 | <0.0001 | −0.144 (0.020) |
| year | 1 | 50.63 | 10.78 | 0.0015 | |
| hatching success | |||||
| year | 1 | 2.47 | 16.62 | <0.0001 | |
| tree species | 1 | 0.91 | 6.13 | 0.016 | −0.37 (0.15) |
| age of stand | 1 | 0.59 | 3.96 | 0.051 | |
| undergrowth | 1 | 0.52 | 3.50 | 0.067 | |
| humidity | 1 | 1.26 | 8.51 | 0.005 | −0.32 (0.11) |
| date | 1 | 0.16 | 1.06 | 0.31 | |
| log (dose rate) | 1 | 0.59 | 3.94 | 0.052 | |
| undergrowth×tree species | 1 | 1.09 | 7.31 | 0.009 | 0.47 (0.18) |
| undergrowth×log (dose rate) | 1 | 1.90 | 12.75 | 0.0007 | 0.89 (0.25) |
| fledging success: | |||||
| age of stand | 1 | 0.04 | 0.22 | 0.64 | |
| undergrowth | 1 | 0.00 | 0.00 | 0.99 | |
| age of stand×undergrowth | 1 | 1.14 | 6.50 | 0.010 | 0.39 (0.15) |
| year | 1 | 1.79 | 10.20 | 0.0025 | |
| pied flycatcher | |||||
| laying date: | |||||
| undergrowth | 1 | 397.34 | 4.25 | 0.049 | −5.79 (2.81) |
| clutch size: | |||||
| date | 1 | 16.93 | 46.82 | <0.0001 | −0.080 (0.012) |
| undergrowth | 1 | 2.39 | 6.62 | 0.016 | −0.48 (0.19) |
| hatching success: | |||||
| date | 1 | 1.93 | 22.56 | <0.0001 | −0.030 (0.006) |
| log (dose rate) | 1 | 0.22 | 2.56 | 0.12 | |
| date×log (dose rate) | 1 | 1.18 | 13.73 | 0.0012 | −0.219 (0.059) |
| year | 1 | 0.35 | 4.03 | 0.06 | |
| fledging success: | |||||
| date | 1 | 0.10 | 4.82 | 0.040 | −0.009 (0.004) |
In analyses of clutch size, we entered laying date as a covariate because clutch size decreased with laying date (table 3). For neither species did dose rate enter as a predictor of clutch size, while there was a significant year effect for great tit and a significant effect of undergrowth for pied flycatcher (table 3).
In the great tit, 13.3% of nests were deserted, 2.9% destroyed by dormice (Eliomys quercinus) and 2.9% by ants (N=105). In the pied flycatcher, 6.2% of nests were deserted (N=32). Hatching success in the great tit differed between years, tree species (lower success in pine) and humidity classes (lower success in humid habitats; table 3). In addition, there was a significant interaction between undergrowth and tree species (implying that hatching success increased with increasing undergrowth for a given tree species) and between undergrowth and dose rate (implying that hatching success increased with increasing undergrowth for a given dose rate; table 3). Hatching success decreased with laying date in pied flycatchers (table 3). In addition, there was a significant interaction between dose rate and laying date, implying that hatching success decreased more with laying date for high levels of radiation (table 3). Fledging success was not significantly related to dose rate in either species, while there were significant effects of the interaction between age of stands and undergrowth in the great tit and a significant effect of date in the pied flycatcher (table 3). We performed partial Mantel tests between reproductive variables and dose rate after controlling for distance among boxes. Only the correlations between hatching success and dose rate were statistically significant (great tit: r=−0.351, p<0.0001; pied flycatcher: r=−0.271, p<0.0001). Entering the other habitat variables in table 3 into these partial Mantel tests did not change the conclusions. Therefore, hatching success was negatively related to dose rate after adjusting for geographical distance.
4. Discussion
The main findings of this study were that (i) the occupation rate of nest boxes decreased with increasing dose rate across a range of background radiation of two orders of magnitude with a stronger effect in pied flycatchers than in great tits; (ii) this effect was not confounded by individuals of a specific age breeding at the most contaminated boxes; (iii) laying date and clutch size was not related to dose rate; and (iv) hatching success decreased with dose rate, interacting with laying date in pied flycatchers and with density of undergrowth in great tits. These findings constitute the first empirical evidence consistent with the suggestion that animals choose sites of reproduction dependent on background radiation levels.
The current study of occupancy of nest sites by hole nesting birds suggested that great tits and pied flycatchers discriminate against nest sites with high dose rates, thereby avoiding radioactively contaminated areas as sites of reproduction. These effects were present in different years. The effects were present even when controlling statistically for the potentially confounding effects of habitat differences covarying with levels of radiation, and there was no evidence that yearlings used boxes with levels of radiation that differed from that used by older birds. The effect for pied flycatchers was considerably stronger than for great tits. To the best of our knowledge, there are no previous study investigating the relationship between radiation and distribution and abundance of plants or animals (Chernobyl Forum 2005; Møller & Mousseau 2006).
Nest-site choice dependent on radioactive contamination begs the question what are the underlying mechanisms? The effect cannot arise from limited ability to disperse because the size of the study area was small when compared with geometric mean natal dispersal distances of 0.8 and 14.3 km in great tits and pied flycatchers (Paradis et al. 1998). We can also dismiss the possibility that this effect was mediated by attraction to conspecifics because the findings were similar in both years (the interaction between year and dose rate was not statistically significant for either species). This implies that the effect was already present when the boxes had never been used and hence did not have signs of previous occupation. We can also dismiss the explanation that the effect was mediated by resource abundance because such an effect would assume that food abundance decreased with increasing dose rate. If that were the case, we would expect clutch size and brood size to decrease with dose rate, which was clearly not observed (table 3). This leaves the possibility that the effect was mediated by differences in individual body condition. We have previously shown for barn swallows Hirundo rustica that individuals in highly contaminated areas have reduced levels of antioxidants in plasma and liver (Møller et al. 2005a). Such individuals have reduced body condition, reproduce less frequently, and produce reduced clutch and brood size when compared with individuals breeding in highly contaminated areas (Møller et al. 2005b). Therefore, we hypothesize a similar mechanism for hole nesting species, with individuals breeding in highly contaminated areas suffering from reduced antioxidant levels, thereby reducing the frequency of reproduction. If individuals differ in condition, with late breeding individuals suffering more from poor condition than early breeders, then we would expect dose effects to be more severe late during the breeding season (predicting an interaction between laying date and dose rate for reproductive variables). Indeed, we found such effects for hatching success in the pied flycatcher (table 3), consistent with this interpretation.
The two species differed in response to dose rate with the pied flycatcher showing a stronger relationship between occupation rate and dose rate than the great tit. Therefore, pied flycatchers bred at sites that were significantly less contaminated than did great tits, reducing the possibility of finding significant effects of radiation on life-history variables in pied flycatchers. Neither laying date nor clutch size was significantly related to dose rate. In contrast, hatching success decreased with dose rate, with a significant interaction with undergrowth cover in the great tit and with laying date in the pied flycatcher. Dose rate did not predict fledging success in either species, suggesting that radioactive contamination did not limit food supply. The two species differ in a number of respects including foraging ecology (great tits mainly eat caterpillars, pied flycatchers by catching flying insects in the air) and migration strategy (tits are residents, while pied flycatchers are long-distance migrants wintering in sub-Saharan Africa). Differences in relationships between occupation rate and dose rate could be due to differences in levels of contamination of food. However, our estimates of whole body dose rates for the two species have revealed very similar mean dose rates (S. Gaschack, A. P. Møller and T. A. Mousseau unpublished data), suggesting that this is an unlikely explanation. In addition, the lack of an association between fledging success and dose rate contradicts that explanation. Alternatively, strong effects in pied flycatchers may arise from this species being migratory arriving on spring migration to the breeding grounds with depleted reserves of antioxidants (Ninni et al. 2004). This depletion of antioxidant reserves may be further exacerbated by extensive maternal deposition of carotenoids and vitamins A and E into eggs (Blount et al. 2000; Blount et al. 2003). Therefore, pied flycatchers would be expected to have lower rates of reproduction than great tits, but also to be selected to show stronger avoidance of breeding sites with low availability of antioxidants.
This study has implications for future studies. First, it would be crucial to determine the association between dose rate and condition of hole nesting passerines in order to test for the hypothesized mechanism. Second, we consider that it would be important to establish a monitoring scheme for a number of common invertebrates and vertebrates to assess to which extent elevated levels of radioactive contamination are associated with reduced population density. Finally, it would be important to combine such studies of distribution and abundance with studies of population dynamics to assess the extent to which apparently high population densities are maintained by source–sink dynamics (Møller et al. 2006). If that were the case, we would expect a larger fraction of immigrant juveniles in the most contaminated areas. The lack of significant association between age of breeders and radiation level and the lack of interaction between species and age suggests that neither great tits nor flycatchers have populations in Chernobyl maintained by source-sink dynamics.
Acknowledgments
We are grateful to G. Milinevski, S. Gaschak, A. M. Peklo, E. Pysanets and M. Bondarkov for logistic help. We received funding from CNRS (France), University of South Carolina School of the Environment, Bill Murray and Samuel Freeman Charitable Trust, National Science Foundation and National Geographic Society for this research.
References
- Addinsoft. Addinsoft; New York, NY: 2006. XLStat. [Google Scholar]
- Anonymous. OECD; Paris: 1996. Chernobyl. Ten years on. Radiological and health impact. [Google Scholar]
- Bazhan K.V. Lipid peroxidation and the antioxidant system in subjects exposed to the influence of extreme factors. Lik Sprava. 1998;Dec(8):47–50. [In Ukrainian.] [PubMed] [Google Scholar]
- Ben-Amotz A, Yatziv S, Sela M, Greenberg S, Rachmilevich B, Shwarzman M, Weshler Z. Effect of natural beta-carotene supplementation in children exposed to radiation from the Chernobyl accident. Radiat. Environ. Biophys. 1998;37:187–193. doi: 10.1007/s004110050116. doi:10.1007/s004110050116 [DOI] [PubMed] [Google Scholar]
- Blount J.D, Houston D.C, Møller A.P. Why egg yolk is yellow. Trends Ecol. Evol. 2000;17:47–49. doi: 10.1016/s0169-5347(99)01774-7. doi:10.1016/S0169-5347(99)01774-7 [DOI] [PubMed] [Google Scholar]
- Blount J.D, Houston D.C, Surai P.F, Møller A.P. Egg-laying capacity is limited by carotenoid pigment availability in wild gulls Larus fuscus. Proc. R. Soc. B. 2003;271:S79–S81. doi: 10.1098/rsbl.2003.0104. doi:10.1098/rsbl.2003.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulinier T, Danchin E. The use of conspecific reproductive success for breeding patch selection in territorial migratory species. Evol. Ecol. 1997;11:505–517. doi:10.1007/s10682-997-1507-0 [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2002. Model selection and multi-model inference. [Google Scholar]
- Chaialo P.P, Bereza V.I, Chobot'ko G.M. Free-radical processes and blood antioxidant systems in the late period following acute radiation sickness. Med. Radiol. (Moscow) 1991;36:20–21. [In Russian.] [PubMed] [Google Scholar]
- Chernobyl Forum. IAEA, WHO, UNDP; Vienna: 2005. Chernobyl: the true scale of the accident. 20 years later a UN report provides definitive answers and ways to repair lives. [Google Scholar]
- Cramp S, Perrins C.M, editors. The birds of the Western Palearctic. Vols 5–7. Oxford University Press; Oxford, UK: 1988–1993. [Google Scholar]
- Danchin E, Wagner R.H. The evolution of coloniality: the emergence of new perspectives. Trends Ecol. Evol. 1997;12:342–347. doi: 10.1016/s0169-5347(97)01124-5. doi:10.1016/S0169-5347(97)01124-5 [DOI] [PubMed] [Google Scholar]
- Fretwell S.D, Lucas J.H.J. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1970;19:16–36. doi:10.1007/BF01601953 [Google Scholar]
- Ivaniota L, Dubchak A.S, Tyshchenko V.K. Free radical oxidation of lipids and antioxidant system of blood in infertile women in a radioactive environment. Ukrainski Biokhim. Zh. 1998;70:132–135. [In Ukrainian.] [PubMed] [Google Scholar]
- Kumerova A.O, Lece A.G, Skesters A.P, Orlikov G.A, Seleznev J.V, Rainsford K.D. Antioxidant defense and trace element imbalance in patients with postradiation syndrome: first report on phase I studies. Biol. Trace Elem. Res. 2000;77:1–12. doi: 10.1385/BTER:77:1:1. doi:10.1385/BTER:77:1:1 [DOI] [PubMed] [Google Scholar]
- Lundberg A, Alatalo R.V. T. & A. D. Poyser; London, UK: 1992. The pied flycatcher. [Google Scholar]
- Lykholat E.A, Chernaya V.I. Parameters of peroxidation and proteolysis in the organism of the liquidators of Chernobyl accident consequences. Ukrainski Biokhim. Zh. 1999;71:82–85. [PubMed] [Google Scholar]
- Møller A.P, Mousseau T.A. Biological consequences of Chernobyl: 20 years after the disaster. Trends Ecol. Evol. 2006;21:200–207. doi: 10.1016/j.tree.2006.01.008. doi:10.1016/j.tree.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Møller A.P, Mousseau T.A, Milinevsky G, Peklo A, Pysanets E, Szép T. Condition, reproduction and survival of barn swallows from Chernobyl. J. Anim. Ecol. 2005a;74:1102–1111. doi:10.1111/j.1365-2656.2005.01009.x [Google Scholar]
- Møller A.P, Surai P.F, Mousseau T.A. Antioxidants, radiation and mutation in barn swallows from Chernobyl. Proc. R. Soc. B. 2005b;272:247–253. doi: 10.1098/rspb.2004.2914. doi:10.1098/rspb.2004.2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A.P, Hobson K.A, Mousseau T.A, Peklo A.M. Chernobyl as a population sink for barn swallows: tracking dispersal using stable isotope profiles. Ecol. Appl. 2006;16:1696–1705. doi: 10.1890/1051-0761(2006)016[1696:caapsf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Nelson N, Shetopalov V. Don't underestimate the death rate from Chernobyl. Nature. 2005;437:1089. doi: 10.1038/4371089a. doi:10.1038/4371089a [DOI] [PubMed] [Google Scholar]
- Neel J.V, Satoh C, Goriki K, Asakawa J, Fujita M, Takahashi N, Kageoka T, Hazama R. Search for mutations altering protein change and/or function in children of atomic bomb survivors: Final report. Am. J. Hum. Genet. 1988;42:663–676. [PMC free article] [PubMed] [Google Scholar]
- Neyfakh E.A, Alimbekova A.I, Ivanenko G.F. Vitamin E and A deficiencies in children correlate with Chernobyl radiation loads of their mothers. Biochemistry (Moscow) 1998a;63:1138–1143. [PubMed] [Google Scholar]
- Neyfakh E.A, Alimbekova A.I, Ivanenko G.F. Radiation-induced lipoperoxidative stress in children coupled with deficit of essential antioxidants. Biochemistry (Moscow) 1998b;63:977–987. [PubMed] [Google Scholar]
- Ninni P, de Lope F, Saino N, Haussy C, Møller A.P. Antioxidants and condition-dependence of arrival date in a migratory passerine. Oikos. 2004;105:55–64. doi:10.1111/j.0030-1299.2004.12516.x [Google Scholar]
- Paradis E, Baillie S.R, Sutherland W.J, Gregory R.D. Patterns of natal and breeding dispersal in birds. J. Anim. Ecol. 1998;67:518–536. doi:10.1046/j.1365-2656.1998.00215.x [Google Scholar]
- Perrins C.M. Collins; London: 1979. British tits. [Google Scholar]
- Shestopalov V.M. Ukrainian Academy of Science; Kiev: 1996. Atlas of Chernobyl exclusion zone. [Google Scholar]
- Smouse P.E, Long J.C, Sokal R.R. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst. Zool. 1986;35:627–632. doi:10.2307/2413122 [Google Scholar]
- Svensson L. 2nd edn. L. Svensson; Stockholm: 1984. Identification guide to European passerines. [Google Scholar]