Abstract
Juvenile cuttlefish (Sepia officinalis) camouflage themselves by changing their body pattern according to the background. This behaviour can be used to investigate visual perception in these molluscs and may also give insight into camouflage design. Edge detection is an important aspect of vision, and here we compare the body patterns that cuttlefish produced in response to checkerboard backgrounds with responses to backgrounds that have the same spatial frequency power spectrum as the checkerboards, but randomized spatial phase. For humans, phase randomization removes visual edges. To describe the cuttlefish body patterns, we scored the level of expression of 20 separate pattern ‘components’, and then derived principal components (PCs) from these scores. After varimax rotation, the first component (PC1) corresponded closely to the so-called disruptive body pattern, and the second (PC2) to the mottle pattern. PC1 was predominantly expressed on checkerboards, and PC2 on phase-randomized backgrounds. Thus, cuttlefish probably have edge detectors that control the expression of disruptive pattern. Although the experiments used unnatural backgrounds, it seems probable that cuttlefish display disruptive camouflage when there are edges in the visual background caused by discrete objects such as pebbles. We discuss the implications of these findings for our understanding of disruptive camouflage.
Keywords: Sepia, cephalopod, camouflage, vision, texture
1. Introduction
Like many cephalopod molluscs, cuttlefish change their body patterns for communication and camouflage (Hanlon & Messenger 1988, 1996; Adamo & Hanlon 1996; Boal et al. 2004; Adamo et al. 2006; Palmer et al. 2006). In camouflage behaviour, visual information from the immediate background affects the expression of coloration patterns (Holmes 1940; Hanlon & Messenger 1988, 1996). Juvenile cuttlefish (Sepia officinalis), if undisturbed, select a pattern that is (apparently) cryptic. The body patterns are produced by coordinated expression of over 30 elements or ‘components’ (Hanlon & Messenger 1988). Flexible expression of these components gives a potentially vast range of possible patterns (Crook et al. 2002); however, Hanlon & Messenger (1988) identify four main types of camouflage that are used by cuttlefish, which they name as ‘uniform’, ‘stipple’, ‘mottle’ and ‘disruptive’.
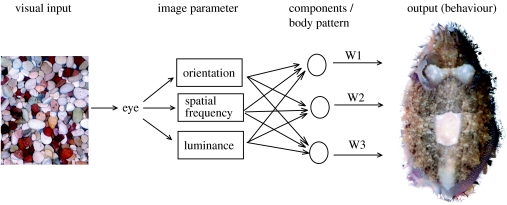
Cuttlefish camouflage gives a unique opportunity to investigate vision in a non-human animal. It is reasonable to assume that when they are on natural backgrounds, the animals select a pattern that gives effective camouflage. To produce a suitable pattern, the cuttlefish needs to be sensitive to measures of the background pattern that are relevant to the vision of natural predators and prey (Osorio & Srinivasan 1991; Portilla & Simoncelli 2000; Victor et al. 2005). One can then test the animal's texture perception by determining how a given image parameter in the substrate affects the pattern expressed (figure 1). Equally, the pattern that is expressed on a given background may give insight into camouflage design. This paper investigates edge detection by cuttlefish and goes on to discuss the use of disruptive camouflage.
Figure 1.
Schematic model of how cuttlefish control their appearance. We propose that the cuttlefish measures a limited number of image parameters (those listed are hypothetical but plausible, Chiao & Hanlon 2001a; Shohet et al. 2006, in press). The values of these parameters drive a motor system that is organized into a set of modules that correspond to the 54 specific pattern components and/or the 12 or more major body patterns W1–3 refer to the weightings or levels of expression of each component or body pattern (Hanlon & Messenger 1988). The cuttlefish is illustrated using a mixture of disruptive and mottle body patterns on the gravel. The image is isolated from its background, as was also done for the blind scoring in this study.
There is no clear definition of disruptive camouflage (see §4), but the term is applied to patterns where high visual contrast elements are thought to interfere with image segmentation (Cott 1940; Merilaita 1998; Endler 2006; Stevens & Cuthill 2006; Stevens et al. 2006a,b). Recent experiments (Cuthill et al. 2005; Schaefer & Stobbe 2006; Stevens et al. 2006b) suggest that for model ‘moths’, which are subject to bird predation, disruptive camouflage is effective over a greater range of backgrounds than is simple crypsis. With cuttlefish one can ask what pattern is used when there is a choice (figures 1 and 3; Hanlon & Messenger 1988; Mäthger et al. 2006; but see Stevens et al. 2006a and §4).
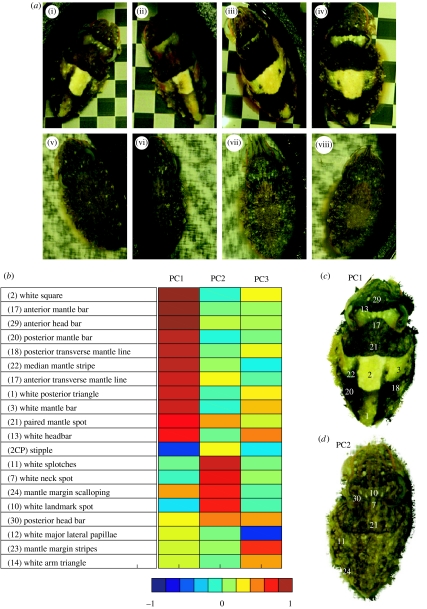
Figure 3.
Body patterns expressed by each of the four experimental cuttlefish, and principal components (PCs) used to analyse the effect of background on the body pattern. PCs were derived with varimax rotation from 20 patterns and skin-texture components (Hanlon & Messenger 1988) that were recorded for the 216 images used in this study. Note that ‘component’ in the latter sense corresponds to an element of motor control. Typically, this is a local visual feature such as the white square, but some features are expressed across the body surface such as skin papillae. (a) Pattern variation between each of the four cuttlefish on background of nominal intensities (i–iv) 0.35 for the conventional checkerboard backgrounds and (v–viii) 0.25 for the phase-randomized backgrounds. (i, ii) and (v, vi) the two smaller cuttlefish and (iii, iv) and (vii, viii) the two larger animals are shown The general difference in responses to these two types of background is clear. Responses to conventional checkerboards at this contrast vary most in the level of expression of the white posterior triangle (1), white mantle bars (3) and white head bar (13) (figure 3b). For the phase-randomized backgrounds, there is very little variation between individual components; the overall expression of the total body pattern varies slightly. (b) The rotated component matrix table shows the loadings of each variable (chromatic or textural component) onto each principal component (PC1, PC2 and PC3). (c) An image of a cuttlefish displaying a disruptive pattern, illustrating the components that load strongly and positively onto PC1 (dark red to orange blocks). (d) An image of a cuttlefish displaying a mottle pattern illustrating the components that load strongly and positively onto PC2. The components loading highly onto PC3 are not part of any single body pattern (Hanlon & Messenger 1988), but are mostly white. The numbered components correspond to those identified by Hanlon and Messenger (Hanlon & Messenger 1998. Crook et al. 2002 and Langridge 2006 reproduce the original figure).
We start from the proposition that cuttlefish map a visual stimulus into a behavioural response (figure 1). However, describing this mapping is not straightforward, because both body patterns and natural backgrounds are difficult to characterize. For humans, the image parameters that identify visual textures are incompletely known (Ruderman & Bialek 1994; Portilla & Simoncelli 2000; Victor et al. 2005). The same problem clearly applies to texture perception in non-human species (Kelman et al. 2006).
In view of the difficulties in characterizing natural visual textures, one can use test patterns that vary in a well-specified way, and ask how low-level image parameters such as spatial frequency, brightness and orientation affect visual behaviour. Previous studies of cuttlefish show that both brightness and spatial frequency affect their body patterns, and that they are colour blind (Hanlon & Messenger 1988; Chiao & Hanlon 2001a; Mäthger et al. 2006; Shohet et al. in press). Orientation does not influence the camouflage pattern, but the animals can sense orientation because they prefer to lie with their body axis across background stripes (Shohet et al. 2006).
One might also expect cuttlefish to be sensitive to the presence of visual edges. However, unlike spatial frequency or contrast, there is no simple definition of a visual edge. One definition is simply that an edge is a local feature that is commonly associated with object borders. The location of visual edges as seen by humans often corresponds to features that have specific spatial phase relationships or ‘phase congruence’ (figure 2; Morrone & Burr 1988). Phase information affects the perceived position of edges and lines, while phase randomization removes them (Morrone & Burr 1988). Spatial phase is also important in characterizing visual texture for human observers (Portilla & Simoncelli 2000; Huang et al. 2006). Despite this evidence, it is not obvious that non-human species should be sensitive to the phase. Humans are relatively insensitive to spatial phase, especially in the peripheral visual field (Rentschler & Treutwein 1985; Huang et al. 2006). Complex (but not simple) cells in cat visual cortex are sensitive to relative phase in natural images (Felsen et al. 2005), but we are unaware of any direct tests of phase sensitivity in a non-mammalian species.
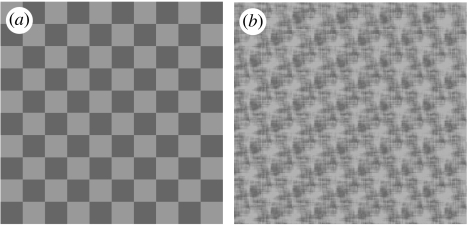
Figure 2.
Examples of backgrounds used to test cuttlefish: (a) conventional checkerboard, with a nominal contrast of 0.15 and (b) the same pattern when phase randomized.
For cuttlefish placed on images of natural backgrounds, low-pass spatial filtering (i.e. blurring) reduces the expression of disruptive body patterns (Chiao et al. 2005). This could be because edges are important, but blurring does not distinguish the effects of modifying the spatial frequency power spectrum from responses to local edges. To resolve this question, we now compare responses to backgrounds that have similar power spectra, but differ in the relative phase of their spatial frequency components (figure 2).
2. Material and methods
(a) Subjects
Four juvenile cuttlefish, Sepia officinalis (L.), (2 of 50 and 2 of 80 mm mantle length) were reared from eggs in the University of Sussex laboratory at the Brighton Sea Life Centre. The animals were maintained under a 12 : 12, L : D lighting regime. They were fed ad libitum with mysids (Mysis spp.) until large enough to accept ghost shrimp (Natantia sp.).
For filming, each animal was transferred individually to a smaller aquarium (900×750 mm, water depth 150 mm). For filming with a horizontally placed camera, a mirror was positioned at an angle of 45° above the tank. To minimize disturbance, the aquarium and mirror were both surrounded with a matte black hood, and filming was done through a small window. Within the filming tank, cuttlefish were restricted to a 250 mm diameter cylindrical arena (with 120 mm black walls). Experimental substrates (figure 2) were placed underneath the arena. When the cuttlefish had settled and expressed a stable body pattern for at least 10 min, they were photographed (Nikon Coolpix 5400).
(b) Stimulus design
The experiment compared responses to conventional checkerboard backgrounds (henceforth ‘checkerboards’; Chiao & Hanlon 2001a), with phase-randomized versions of the same patterns (henceforth ‘phase-randomized patterns’; figure 2). Images were produced with Matlab software, and the patterns printed with a laserjet onto water-resistant transparencies. The prints were calibrated for linearity with a grey scale over a range of reflectance from 0.1 to 0.8.
The primary purpose of this study was to show that animals are sensitive to spatial phase. Hence, to exclude the effects of visual system nonlinearities such as response saturation, checkerboard backgrounds were tested over a range of contrasts (Imax−Imin/Imax+Imin) with nominal values of 1.0, 0.60, 0.35, 0.15, 0.10 and 0.05. Phase-randomized patterns were obtained from checkerboards with contrasts of 1.0, 0.25 and 0.15.
It is thought (Chiao & Hanlon 2001a,b; Mäthger et al. 2006; A. Barbosa & C. C. Chiao, personal communication) that expression of body patterns is dependent on the relationship between the size of ‘light objects’ in the background and the animals' body size. For this reason, the size of the checkerboard squares was approximately equal to the ‘white square’ used in the disruptive pattern (figures 1 and 3). We used two sizes of check: 21×21 mm for the 80 mm animals and 13×13 mm for the 50 mm animals. The four cuttlefish were tested six times each on nine backgrounds, which were randomized with respect to the order of presentation.
(c) Image quantification
Each of the 216 images collected was graded by eye. To ensure that grading was done blind to the background, the images were extracted from the background using Adobe Photoshop 6.0. Sepia officinalis body patterns have 34 recognized chromatic components (Hanlon & Messenger 1988). These chromatic components are under independent physiological control and can be expressed singly or in various combinations, and produce yellows, orange and browns when chromatophores are expanded and white when the chromatophores are retracted and the underlying reflector cells are operating (Hanlon & Messenger 1988). Although cuttlefish are colour blind (Marshall & Messenger 1996; Mäthger et al. 2006), the patterns are ‘colourful’ to the human eye. ‘Typical’ combinations are known as body patterns (figures 1 and 3–5; Hanlon & Messenger 1988; Crook et al. 2002). Here, the 20 most commonly seen visual components in juvenile S. officinalis (figure 3) were used for grading, with each component graded on a four-point scale (Chiao et al. 2005): 0 (absent) to 3 (strongly expressed).
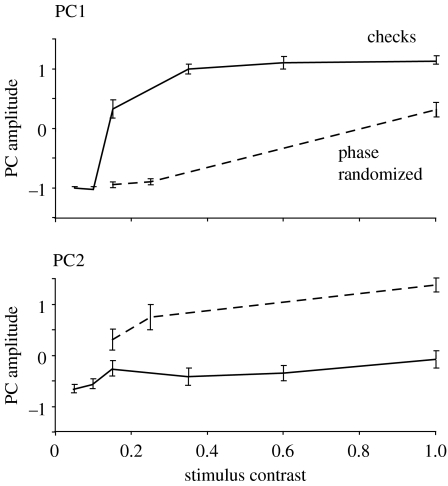
Figure 5.
Effect of background type and contrast on the expression of the PCs 1 and 2. The mean scores of weights of PC1 (above) and PC2 (below) ±s.e.m. are plotted for each of the nine backgrounds used (six for conventional checkerboards, solid line; and three for phase randomized, dashed line). PC1 is elicited by conventional checkerboards and PC2 by the phase-randomized patterns. An ANOVA shows that the interaction between background and PC amplitude is significant (see §3).
(d) Data analyses
Using the scores for expression of the 20 pattern ‘components’ in each image, we performed a principal component analysis (PCA; SPSS v. 11.5). Following Kaiser's (1960) criterion, only components with eigenvalues exceeding one were retained. To facilitate interpretation, the components were subjected to a varimax rotation. This selects a set of axes in the components' subspace that maximizes the variance of their loadings. This rotation was effective in that the values of basis functions were sensitive to the experimental treatment, and also the first two principal components (PCs) could readily be related to recognized body patterns: PC1 with ‘disruptive’ and PC2 with ‘mottle’ (figures 3–5; Hanlon & Messenger 1988). Variables (i.e. chromatic components) with loading 0.40 or more on a particular factor and sharing at least 15% of the variance with the factor were used for the ease of interpreting the outcome (Stevens 1992). SPSS used this loading value as a default value, which proved useful for our results.
Figure 4.
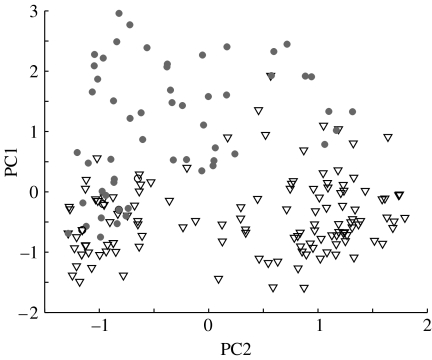
Evidence that cuttlefish can vary the expression of PCs 1 and 2 independently to produce a wide range of patterns. Weightings of PC1 versus PC2 (figure 3) for all 216 images scored in this study. Filled circles, responses to standard checkerboards; triangles, responses to phase-randomized patterns.
The mean loadings for each PC were calculated by averaging the scores for each cuttlefish over all examples of the given background. These means were used to determine the effects of background on the coloration pattern expressed.
To identify interactions between the background context (standard or phase-randomized checkerboard) and the body patterns the cuttlefish produced on these backgrounds, we used a repeated measures ANOVA (SPSS v. 11.5). The fact that the main variance in the expression of body patterns (PC1 and PC2) occurred at the higher contrast values (standard=1, 0.60 and 0.35; and phase randomized=1, and 0.25) justified the exclusion of lower contrast data (figure 5). A mean value of the high-contrast loadings were calculated for each individual cuttlefish and each trial, and these data were then run through the ANOVA.
3. Results
For the 216 cuttlefish body patterns used in the study, three principal components (PCs 1–3) accounted for 66% of the total variance in expression of the 20 behavioural components scored (figure 3). A scree plot indicated that fitting more than three PCs was not meaningful. The first two components could readily be related to the body patterns identified by Hanlon & Messenger (1988): PC1 corresponds to the disruptive pattern and PC2 to mottle (figure 3c,d).
The weights of all three PCs increased with background contrast, and the relative weights of PCs 1 and 2 were clearly dependent upon the background (figures 3a and 5). PC1 was produced mainly in response to checkerboards and PC2 to the phase-randomized patterns. With combined responses for the high-contrast backgrounds (see §2), an ANOVA showed a significant effect of the background type (checkerboard or phase randomized) with the expression of PCs 1 and 2 (F(1,23)=495.52, p<0.05). Thus, the cuttlefish's choice of body pattern is dependent on spatial phase information in the background upon which it is resting. In addition, the cuttlefish can vary levels of expression of PCs 1 and 2 independently, giving them flexibility in their overall appearance (figures 1 and 4; Kelman et al. 2006).
Given that the backgrounds were varied on only two dimensions, one would not expect more than two PCs in the response. However, PC3 appeared to be meaningful; it reflects the expression of several white pattern components: white head bar; white mantle bar; and the white arm triangle; and also a rarely used dark mantle margin stripe (figure 3b). The expression of PC3 appeared to be strongest over high-contrast checkerboards, but we did not analyse its expression further.
4. Discussion
These results demonstrate that the cuttlefish S. officinalis are sensitive to spatial phase (figures 2 and 5). On a uniform background, the animals produce a relatively uniform body pattern. Increasing contrast of a conventional checkerboard drives the expression of disruptive pattern (identified here with PC1), whereas increasing contrast of a phase-randomized checkerboard drives the expression of mottle pattern (identified with PC2). These findings are consistent with the observation that blurring reduces the expression of disruptive pattern (Chiao et al. 2005), and suggest that it is the effect of blurring on local image features, rather than power at high spatial frequencies that underlies this observation.
Given that phase randomization removes information about the location of edges, there are two main conclusions: first, that cuttlefish have an edge detector that is sensitive to local spatial structure (figure 5; Rentschler & Treutwein 1985; Morrone & Burr 1988; Huang et al. 2006) and second, that the presence of edges disposes the animal to produce the disruptive pattern (Hanlon & Messenger 1988). This pattern is, of course, characterized by obvious visual edges (figure 3). It should be emphasized that the particular body pattern that is produced for a given background will be affected by many factors that are not investigated here, e.g. the size, shape, contrast polarity and density of the objects of interest (Chiao & Hanlon 2001a,b; Chiao et al. 2005).
As we discuss below, disruptive camouflage is often identified by the presence of visual contrasts in a coloration pattern which are higher than those of the background. It is therefore of interest to ask how the cuttlefish adjusts the contrast of its body pattern relative to that of the background. Previous work shows that the contrast in a checkerboard background affects the level of expression of disruptive patterns (Chiao & Hanlon 2001a,b; Mäthger et al. 2006). We did not set out to test contrast sensitivity, but there is an indication that the expression of PC1 on a checkerboard rises between pattern contrasts of 0.1 and 0.4, and then saturates (figure 5). For comparison, the contrast between light and dark regions in the disruptive pattern (figure 3a,c) is approximately 0.6 (unpublished observations), which is a value that would correspond to a high reflectance contrast for natural substrate materials. Although further work is needed, the implication is that in cuttlefish, the contrast in the disruptive body pattern is approximately equal to that in the background.
(a) What is disruptive camouflage?
When cuttlefish settle on a background of relatively large discrete objects, such as pebbles, they see edges, and it is not surprising that they produce a camouflage pattern that includes large features separated by clear borders (figure 3). This is called a disruptive body pattern (Hanlon & Messenger 1988). The term is used because it seems probable that the pattern produces disruptive camouflage, which Cott (1940) defines as interfering with object recognition by ‘uncoupling, visually, a part of the body to the rest and by creating false lines and edges’ (see also Merilaita 1998; Stevens et al. 2006a). An example is the high-contrast edges of the white band on cuttlefish, which runs perpendicular to the edge of the mantle (Cott 1940, p. 95). A different disruptive principle is illustrated by the white square (figure 3), which can be shaded asymmetrically to resemble a pebble lit from the side (Anderson et al. 2003; Langridge 2006). This shading has the effect of destroying the integrity of the body surface in a two-dimensional plane.
The versatility of the cuttlefish coloration is demonstrated by their ability to mix the main types of body pattern (figures 1 and 4), and to produce variation within these basic patterns (Hanlon & Messenger 1988; Crook et al. 2002). By comparison, flatfish such as plaice (Pleuronectes platessa) mix a small number (1, 2 or 3 according to species) of basic patterns, but cannot fine-tune those patterns (Kelman et al. 2006). The exceptional ability of cephalopods promises insights into how camouflage operates, and in particular the significance of disruptive patterns. This can include, but is not restricted to, disruption of the animal's outline. Field studies by Cuthill and others (Cuthill et al. 2005; Schaefer & Stobbe 2006; Stevens et al. 2006b) suggest that disruptive coloration is effective; it was shown that for model moths placed on tree bark, high-contrast features reduce ‘predation’ most effectively when they intersect with the outline. Interestingly, optimal concealment of model moths is achieved by contrasts that are not in excess of those in the background (Stevens et al. 2006b).
The idea that disruptive coloration works by disrupting image segmentation (and hence object detection) has led some authors to distinguish disruptive camouflage from crypsis, which is in direct resemblance to the background (Endler 1978, 2006; Merilaita 1998; Stevens et al. 2006a). Disruptive camouflage is then identified by the presence unnaturally high visual contrasts in the body pattern. It follows from this argument that cuttlefish's ‘disruptive patterns’ are not proven to be disruptive camouflage: firstly, because there is no evidence that they interfere with figure–ground segregation and secondly, because the contrast in the body pattern is not higher than that in the background (Ruxton et al. 2004; Stevens et al. 2006a). The observations here support this argument in that the disruptive body pattern is used when it is cryptic, i.e. when there are edges in the background, but not otherwise. Also, up to a background contrast of approximately 0.5, the level of expression of the disruptive pattern appears to reflect the contrast in the background (figure 5; Mäthger et al. 2006)—higher reflectance contrasts are relatively rare in nature.
The interpretation that the disruptive pattern is primarily cryptic is strengthened by the finding that display of the white square (figure 3), which is a component of this pattern, is facilitated by the presence of visual features in the background that have a similar size to this component (Chiao & Hanlon 2001b). Moreover, Chiao & Hanlon (2001b) found that it is the area of the light objects on a dark background that is crucial for eliciting the expression of the white square. Thus, cuttlefish camouflage is consistent with the experimental finding that (for avian predation) camouflage is most effective when general matching of the background intensity and colour is combined with disruptive elements whose contrast is not in excess of those in the background (Stevens et al. 2006b).
If the cuttlefish disruptive body pattern is indeed effectively cryptic (Endler 1978), can it also be disruptive in the sense of Cott (1940) and others (Merilaita 1998; Endler 2006; Stevens et al. 2006a)? As we have noted, there are two distinct definitions of disruptive camouflage: one that draws a distinction between general resemblance and disruptive camouflage (Ruxton et al. 2004; Endler 2006; Stevens et al. 2006a), and one that does not (Hanlon & Messenger 1988, and also Stevens et al. 2006b). The distinction seems to us to be relevant mainly when the animal is smaller than the objects among which it is concealed, as for a moth on a tree trunk (Cuthill et al. 2005). When a background includes small objects, as for a cuttlefish among pebbles, a cryptic pattern should include edges, and one may expect them in the interests of camouflage, to be placed disruptively (Merilaita 1998). Given that natural backgrounds do not resemble our experimental stimuli (figure 2), it remains an open question how the cuttlefish respond in more realistic conditions where objects are of varying size and shape (Chiao & Hanlon 2001b; Chiao et al. 2005).
Further difficulties with distinguishing between cryptic and disruptive patterns lie in the definition of crypsis as a match to the background (Endler 1978). This issue is especially pertinent when an animal is concealed among small discrete objects (e.g. pebbles). Firstly, a close textural match does not necessarily give good camouflage, for example, if the body pattern does not align perfectly with well-defined features in the background, such as edges (Shohet et al. 2006—figure 5). More fundamentally, ‘matching’ needs to be defined with respect to a specific visual filter or neural representation, but the relevant description is unknown. Despite recent advances (Portilla & Simoncelli 2000), there is no reliable basis for predicting whether two visual textures will match for human vision, much less for other animals (see §1). Conversely, patterns that appear to be a poor match for any natural stimulus, such as the enhanced edges that are found in disruptive patterns, may be indistinguishable from the natural stimulus for relevant visual mechanisms, in this case edge detectors (Osorio & Srinivasan 1991; Stevens & Cuthill 2006). Lastly, high contrasts in natural images are often caused by cast shadows or holes. The absence of high-contrast borders in reflectance of the background pattern does not preclude a coloration pattern that includes such borders from being cryptic (Endler 1978); this point is well described by Kipling (1902) in ‘How the leopard got its spots’.
To conclude, we have shown that the presence of localized visual edges is important in causing cuttlefish to produce disruptive, as opposed to mottled, body patterns. In nature, edges are typically caused by discrete objects such as pebbles. Cuttlefish probably use the disruptive body pattern when this allows general resemblance to the surroundings, but the visual features within this pattern are placed disruptively so as to prevent detection of the animal's outline.
Acknowledgments
We thank C. C. Chiao and R. T. Hanlon for their hospitality at Woods Hole Marine Biology Laboratory, and for discussion and advice on cuttlefish visual behaviour.
References
- Adamo S.A, Hanlon R.T. Do cuttlefish (Cephalopoda) signal their intentions to conspecifics during agonistic encounters? Anim. Behav. 1996;52:73–81. doi:10.1006/anbe.1996.0153 [Google Scholar]
- Adamo S.A, Ehgoetz K, Sangster C, Whitehorne I. Signalling to the enemy? Body pattern expression and its response to external cues during hunting in the cuttlefish Sepia officinalis (Cephalopoda) Biol. Bull. 2006;210:192–200. doi: 10.2307/4134557. [DOI] [PubMed] [Google Scholar]
- Anderson J.C, Baddeley R.J, Osorio D, Shashar N, Tyler C.W, Ramachandran V.S, Crook A.C, Hanlon R.T. Modular organisation of adaptive coloration in flounder and cuttlefish revealed by independent component analysis. Network. 2003;14:321–333. [PubMed] [Google Scholar]
- Boal J.G, Shashar N, Grable M.M, Vaughan K.H, Loew E.R, Hanlon R.T. Behavioural evidence for intra-specific signalling with achromatic and polarised light by cuttlefish (Mollusca: Cephalopoda) Behaviour. 2004;141:837–861. doi:10.1163/1568539042265662 [Google Scholar]
- Chiao C.C, Hanlon R.T. Cuttlefish camouflage: visual perception of size contrast and number of white squares on artificial checkerboard substrate initiates disruptive coloration. J. Exp. Biol. 2001a;204:2119–2125. doi: 10.1242/jeb.204.12.2119. [DOI] [PubMed] [Google Scholar]
- Chiao C.C, Hanlon R.T. Cuttlefish cue visually on area—not shape or aspect ratio—of light objects in the substrate to produce disruptive body patterns for camouflage. Biol. Bull. 2001b;201:269–270. doi: 10.2307/1543359. doi:10.2307/1543359 [DOI] [PubMed] [Google Scholar]
- Chiao C.C, Kelman E.J, Hanlon R.T. Disruptive body patterning of cuttlefish (Sepia officinalis) require visual information regarding edges and contrast of objects in natural substrate backgrounds. Biol. Bull. 2005;208:7–11. doi: 10.2307/3593095. doi:10.2307/3593095 [DOI] [PubMed] [Google Scholar]
- Cott H.B. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Crook A.C, Baddeley R, Osorio D. Identifying the structure in cuttlefish visual signals. Phil. Trans. R. Soc. B. 2002;357:1617–1624. doi: 10.1098/rstb.2002.1070. doi:10.1098/rstb.2002.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill I.C, Stevens M, Sheppard J, Maddocks T, Párraga C.A, Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Endler J.A. A predator's view of animal color patterns. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Endler J.A. Disruptive and cryptic coloration. Proc. R. Soc. B. 2006;273:2425–2426. doi: 10.1098/rspb.2006.3650. doi:10.1098/rspb.2006.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsen G, Touryan J, Han F, Dan Y. Cortical sensitivity to visual features in natural scenes. PLoS Biol. 2005;3:e342. doi: 10.1371/journal.pbio.0030342. doi:10.1371/journal.pbio.0030342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon R.T, Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body pattern and their relation to behaviour. Phil. Trans. R. Soc. B. 1988;320:437–487. doi:10.1098/rstb.1988.0087 [Google Scholar]
- Hanlon R.T, Messenger J.B. Cambridge University Press; Cambridge, UK: 1996. Cephalopod behaviour. [Google Scholar]
- Holmes W. The colour changes and colour patterns of Sepia officinalis L. Proc. Zool. Soc. Lond. 1940;110:2–35. [Google Scholar]
- Huang P.C, Kingdom F.A.A, Hess R.F. Only two phase mechanisms, cosine plus and minus, in human vision. Vision Res. 2006;46:2069–2081. doi: 10.1016/j.visres.2005.12.020. doi:10.1016/j.visres.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Kaiser H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika. 1960;23:187–200. doi:10.1007/BF02289233 [Google Scholar]
- Kelman E.J, Tiptus P, Osorio D. Juvenile plaice (Pleuronectes platessa) produce camouflage by flexibly combining two separate patterns. J. Exp. Biol. 2006;209:3288–3292. doi: 10.1242/jeb.02380. doi:10.1242/jeb.02380 [DOI] [PubMed] [Google Scholar]
- Kipling R. Macmillan; London, UK: 1902. Just so stories for little children: how the leopard got its spots. [Google Scholar]
- Langridge K.V. Symmetrical crypsis and asymmetrical signalling in the cuttlefish Sepia officinalis. Proc. R. Soc. B. 2006;273:959–967. doi: 10.1098/rspb.2005.3395. doi:10.1098/rspb.2005.3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.J, Messenger J.B. Colour-blind camouflage. Nature. 1996;382:408–409. doi:10.1038/382408b0 [Google Scholar]
- Mäthger L.M, Barbosa A, Miner S, Hanlon R.T. Colour blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vision Res. 2006;46:1746–1753. doi: 10.1016/j.visres.2005.09.035. doi:10.1016/j.visres.2005.09.035 [DOI] [PubMed] [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. B. 1998;265:1059–1064. doi:10.1098/rspb.1998.0399 [Google Scholar]
- Morrone M.C, Burr D.C. Feature detection in human vision: a phase dependent energy model. Proc. R. Soc. B. 1988;235:221–245. doi: 10.1098/rspb.1988.0073. [DOI] [PubMed] [Google Scholar]
- Osorio D, Srinivasan M.V. Camouflage by edge enhancement in animal coloration patterns and its implications for visual mechanisms. Proc. R. Soc. B. 1991;244:81–85. doi: 10.1098/rspb.1991.0054. doi:10.1098/rspb.1991.0054 [DOI] [PubMed] [Google Scholar]
- Palmer M.E, Calvé M.R, Adamo S.A. Response of female cuttlefish Sepia officinalis (Cephalopoda) to mirrors and conspecifics: evidence for signalling in female cuttlefish. Anim. Cogn. 2006;9:151–155. doi: 10.1007/s10071-005-0009-0. doi:10.1007/s10071-005-0009-0 [DOI] [PubMed] [Google Scholar]
- Portilla J, Simoncelli E.P. A parametric texture model based on joint statistics of complex wavelet coefficients. Int. J. Comp. Vision. 2000;40:49–71. doi:10.1023/A:1026553619983 [Google Scholar]
- Rentschler I, Treutwein B. Loss of spatial phase relationships in extrafoveal vision. Nature. 1985;313:308–310. doi: 10.1038/313308a0. doi:10.1038/313308a0 [DOI] [PubMed] [Google Scholar]
- Ruderman D.L, Bialek W. Statistics of natural images: scaling in the woods. Phys. Rev. Lett. 1994;73:814–817. doi: 10.1103/PhysRevLett.73.814. doi:10.1103/PhysRevLett.73.814 [DOI] [PubMed] [Google Scholar]
- Ruxton G.D, Sherratt T.N, Speed M.P. Oxford University Press; Oxford, UK: 2004. Avoiding attack. [Google Scholar]
- Schaefer H.M, Stobbe N. Disruptive coloration provides camouflage independent of background matching. Proc. R. Soc. B. 2006;273:2427–2432. doi: 10.1098/rspb.2006.3615. doi:10.1098/rspb.2006.3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet A.J, Baddeley R.J, Anderson J.C, Kelman E.J, Osorio D. Cuttlefish response to visual orientation of substrates, water flow and a model of motion camouflage. J. Exp. Biol. 2006;209:4717–4723. doi: 10.1242/jeb.02580. doi:10.1242/jeb.02580 [DOI] [PubMed] [Google Scholar]
- Shohet, A. J., Baddeley, R. J., Anderson, J. C., & Osorio, D. In press. The visual ecology of cuttlefish camouflage: a quantitative study of species differences. Biol. J. Linn. Soc
- Stevens J.P. 2nd edn. Erlbaum; Hillsdale, NJ: 1992. Applied multivariate statistics for the social sciences. [Google Scholar]
- Stevens M, Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C, Parraga C.A, Troscianko T. The effectiveness of disruptive coloration as a concealment strategy. Prog. Brain Res. 2006a;155:49–65. doi: 10.1016/S0079-6123(06)55004-6. [DOI] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C, Windsor A.M.M, Walker H.J. Disruptive contrast in animal camouflage. Proc. R. Soc. B. 2006b;273:2433–2438. doi: 10.1098/rspb.2006.3614. doi:10.1098/rspb.2006.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J.D, Conte M.M, Chubb C.F. Interaction of luminance and higher-order statistics in texture discrimination. Vision Res. 2005;45:311–328. doi: 10.1016/j.visres.2004.08.013. doi:10.1016/j.visres.2004.08.013 [DOI] [PubMed] [Google Scholar]