Abstract
Introductions of non-native species are seen as major threats to ecosystem function and biodiversity. However, invasions of aquatic habitats by non-native species are known to benefit generalist consumers that exhibit dietary switches and prey upon the exotic species in addition to or in preference to native ones. There is, however, little knowledge concerning the population-level implications of such dietary changes. Here, we show that the introduction of the Manila clam Tapes philippinarum into European coastal waters has presented the Eurasian oystercatcher Haematopus ostralegus ostralegus with a new food resource and resulted in a previously unknown predator–prey interaction between these species. We demonstrate, with an individuals-based simulation model, that the presence of this non-native shellfish, even at the current low density, has reduced the predicted over-winter mortality of oystercatchers at one recently invaded site. Further increases in clam population density are predicted to have even more pronounced effects on the density dependence of oystercatcher over-winter mortality. These results suggest that if the Manila clam were to spread around European coastal waters, a process which is likely to be facilitated by global warming, this could have considerable benefits for many shellfish-eating shorebird populations.
Keywords: biological invasions, Manila clam, Tapes philippinarum, climate change
1. Introduction
The five most important determinants of changes in biodiversity at the global scale have been identified as changes in land use, atmospheric CO2 concentration, nitrogen deposition and acid rain, climate change and biotic exchanges i.e. deliberate or accidental introduction of plants and animals to an ecosystem (Sala et al. 2000). Biological invasions are already a major component in global environmental change, leading to changes in economic value, biological diversity, ecosystem function and evolutionary pathways (Mooney & Cleland 2001; Leppäkoski et al. 2002). Most studies dealing with invasions by non-native species focus on the potential or observed detrimental effects (Mooney & Cleland 2001; Leppäkoski et al. 2002; Occipinti-Ambrogi & Savini 2003). However, invasions have the potential to be beneficial in some respects. Invasions of both marine and freshwater habitats by non-native shellfish have presented diving ducks in North America and Europe with additional food resources which have been exploited due to the greater availability and energetic profitability of the non-native species (Hamilton et al. 1994; Richman & Lovvorn 2004; Werner et al. 2005; Leopold et al. in press). The invasion of freshwater lakes in both Europe and North America by the zebra mussel Dreissena polymorpha has caused changes to the migration patterns and dramatic increases in the local abundance of diving ducks (Suter 1982a,b; Wormington & Leach 1992; Stark et al. 1999). Here, we investigate whether the invasion of European coastal waters by the Manila clam (Tapes philippinarum) could have beneficial population-level consequences for shellfish-eating shorebirds.
Manila clams are native to the western Pacific Ocean and first spread from this area between the 1920s and 1940s when introduced to the Hawaiian Islands and the Pacific coasts of Canada and the USA (Goulletquer 1997). Manila clams have since been introduced to several European countries and have naturalized in Italy, France and Britain (Goulletquer & Héral 1997). The most spectacular invasion by Manila clams occurred in the Adriatic Sea following their introduction to Venice Lagoon in 1983 (Breber 2002). Naturally formed beds in the most favoured areas of Venice Lagoon now hold densities of more than 1000 clams m−2 and the species has spread along the Adriatic coast at 30 km year−1 (Breber 2002). Manila clams were deliberately introduced to Britain in the late 1980s for the purpose of aquaculture. This release was conducted in the light of studies which suggested that water temperatures would be too low to support successful larval production or recruitment (Laing & Utting 1994). However, at one site, Poole Harbour in Dorset, Manila clams have naturalized (Jensen et al. 2004, 2005).
The coasts of many western European countries serve as stopover sites and wintering grounds for migratory shorebirds (Smit & Piersma 1989). Here, they rely upon adequate food resources in order to survive the winter and early spring in good enough condition to migrate in time to breed successfully (Goss-Custard 1985). The cold winter of 1962–1963 led to a prolific recruitment of cockles Cerastoderma edule in The Wash in England (Dare et al. 2004). In contrast, a series of mild winters in the late 1980s led to repeated recruitment failure of several shellfish species in the Wadden Sea and resulted in low standing stocks of prey for shorebirds (Beukema 1992). Climate change is predicted to affect the reproduction and lead to a contraction of the range of many ‘northern’ species of macroinvertebrates upon which shorebirds currently feed (Kendall et al. 2004; Lawrence & Soame 2004; Mieszkowska et al. 2006). Thus, any spread of ‘southern’ or non-native shellfish species may be of considerable benefit to shellfish-eating shorebirds. Here, we demonstrate that the naturalized population of Manila clams in Poole Harbour is exploited by the Eurasian oystercatcher (Haematopus ostralegus ostralegus) and describe the details of this novel predator–prey interaction. We use a behaviour-based individuals-based simulation model (IBM) of shorebird foraging (Goss-Custard & Stillman in press) to predict the way in which the density dependence of over-winter mortality of oystercatchers may be altered by Manila clams' presence.
2. Material and methods
(a) Field observations of oystercatchers
Oystercatchers foraging on the intertidal mudflats of Poole Harbour (Lat 50°42′44″ N Lon 2°03′30″ W) were observed on two consecutive days per month between August 2004 and March 2005. Observations over distances of up to 250 m were made from a shore-based observation point using a telescope. Focal individuals were selected at random and observed for two consecutive 5 min periods. Each prey item consumed was identified to species whenever possible and its size assessed on the basis of the length of the shell and/or the volume of flesh ingested.
(b) Dietary and size-selection assessments
Owing to the distance over which observations were made, it was not always possible to positively identify prey items to species level. Birds for which the majority of prey items could be clearly identified as being of one species were classified as specializing on that species of prey. Some of these birds were Manila clam specialists. Among the birds that could not be classified in this way, there were some individuals that consumed Manila clams occasionally as part of a mixed diet. These birds were categorized separately from those specializing on Manila clams. This allowed the number of ‘specialist’ birds to be analysed separately from the total number of birds that included Manila clams in their diet (i.e. the sum of the two groups). The total number of birds including Manila clams in their diet and the number specializing on Manila clams in each month were converted to proportions by dividing by the total number of birds observed in each month.
The size range of Manila clams taken by oystercatchers was assessed on the basis of only those prey items that were lifted clear of the sediment and identified as being Manila clams (i.e. ignoring the lengths of any shellfish of uncertain identity that were consumed by birds known to consume Manila clams). The size range of Manila clams present in the mud was derived by combining monthly samples of clams taken between August 2003 and March 2004 (Humphreys et al. 2007).
(c) Estimating intake rates of the three principal diets
Estimates of prey size based purely on the volume of flesh were converted to estimated shell length on the basis of prey species-specific regression equations derived from occasions on which both measures were recorded. Estimates of shell length were corrected for observer bias by a standard procedure (Goss-Custard et al. 1987). Using standard procedures (West et al. 2003), ash-free dry mass (AFDM)–length relationships were derived from samples of approximately 50 individuals of each of the three principal prey species (Manila clams, cockles and sand-gapers (Mya arenaria)) collected in September 2002 and March 2003. These relationships were used to generate the estimated AFDM (mg) of flesh of each prey consumed, allowing for the rate of seasonal change in flesh content and the date of observation. These values were summed for each 5 min period of observation and used to derive the mean instantaneous intake rate achieved by each focal bird (mg AFDM s−1).
The intake rates achieved by birds specializing in the three principal diets were compared to establish the homogeneity of variances (Levene's test for non-homogeneity of variances; F2,65=0.23, p=0.798). The data conform to the assumption of equal variances.
(d) Modelling
Individuals-based models predict how animal populations will be affected by changes in their environment by modelling the responses of fitness-maximizing individuals to environmental change and by calculating how their aggregate responses change the average fitness of individuals and thus demographic rates of the population (Goss-Custard & Stillman in press). Goss-Custard & Stillman (in press) provide a detailed description of the generic model. The particular model of Poole Harbour used here is described by Durell et al. (2006). The methods used to survey the numerical and biomass density of the intertidal macrozoobenthos throughout Poole Harbour in order to parametrize the model are described by Caldow et al. (2005). The stable over-winter population of oystercatchers in Poole Harbour is approximately 1200 (Durell et al. 2006). In exploring the density-dependent mortality function, we used populations of between 500 and 6000 birds, i.e. allowing for a five- to sixfold increase in population size. Populations of oystercatchers were varied while holding the populations of the other principal wader species in the harbour, i.e. dunlin Calidris alpina, redshank Tringa totanus, black-tailed godwit Limosa limosa and curlew Numenius arquata at present day values. As our field observations to date have focused on oystercatchers alone, only they were allowed to consume Manila clams in the model.
Simulations were run in which the prey resources available in the model (i) did not include Manila clams, (ii) included the current population of Manila clams in each of the intertidal patches within the harbour, and (iii) simulated proportionate increases to the clam density in each patch so as to achieve harbour-wide average densities of 10, 20 and 40 clams m−2. In all simulations in which Manila clams were included, the clam population structure was assumed to be the same as that already present in the harbour (Humphreys et al. 2007).
The principal output under each scenario is the percentage of the initial autumn population of oystercatchers that is predicted to die due to starvation between September and March.
3. Results
(a) Observations of oystercatcher foraging behaviour
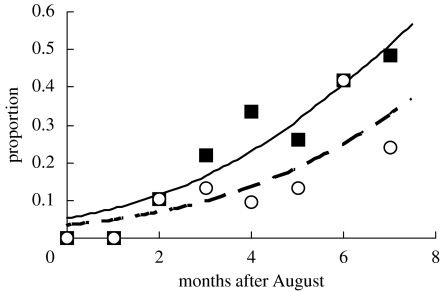
A total of 158 oystercatchers were observed. Of these, it was possible to positively identify the majority of prey items eaten by 86 birds (Manila clams (n=27), cockles (n=17), sand-gapers (n=24), mussels Mytilus edulis (n=3), Macoma balthica (n=5) and worms (n=10)). Of the remaining birds (n=72), three were feeding on prey so small as to be virtually invisible to the observer, 29 were feeding on larger but unidentifiable prey which were probably not bivalves and 40 were feeding on bivalves that, due to factors including the distance of the bird, its particular method of dealing with prey and poor visibility, could not be clearly identified. The proportion of oystercatchers that specialized on feeding on Manila clams increased significantly between late summer and the following spring (figure 1). In addition to the 27 birds that specialized on Manila clams, a further 17 birds were seen to include an occasional Manila clam in their diet. The proportion of all birds that was seen to consume Manila clams (n=44 out of 158) also increased significantly between late summer and the following spring (figure 1).
Figure 1.
Seasonal variation in the proportion of oystercatchers which specialized on feeding on Manila clams (open circles) and of those which ever included Manila clams in their diet (filled squares). The total numbers of birds observed in consecutive months are 7, 12, 19, 23, 21, 23, 24 and 29 (Σ=158). The lines depict the binary logistic regression equations between date and the proportions (p) of birds, specializing on Manila clams (dashed line; log(p/(1−p))=−3.339+0.373* months after August, G=10.92, n=158, d.f.=1, p=0.001), and those including Manila clams in their diet (solid line; log(p/(1−p))=−2.882+0.419* months after August, G=19.41, n=158, d.f.=1, p<0.001).
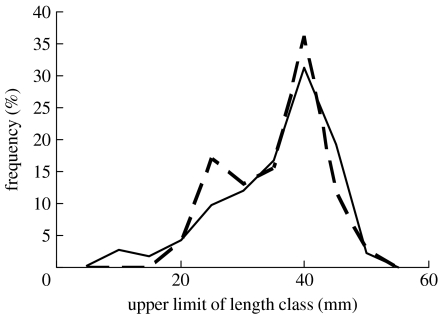
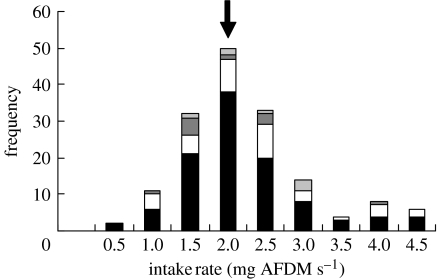
Oystercatchers consumed Manila clams within the length range 16–50 mm. The cumulative frequency distributions of the sizes of clams consumed by oystercatchers and those available in the mud differed significantly (Kolmogorov–Smirnov test D=0.241, n=859, 77; p<0.001), primarily because the birds ignored clams smaller than 15 mm (figure 2). The average intake rate achieved by oystercatchers that specialized in the three principal diets did not differ significantly (ANOVA F2,65=2.18, p=0.1218, Manila clams: mean 2.02 mg AFDM s−1, s.d.=0.90, n=27, cockles: mean 1.81 mg AFDM s−1, s.d.=0.99, n=17 and sand-gapers: mean 1.49 mg AFDM s−1, s.d.=0.83, n=24). The intake rate of Manila clam ‘specialists’ in Poole Harbour compares favourably with that achieved by oystercatchers feeding on native bivalve species not only within Poole Harbour, but also at many other sites around Europe (figure 3).
Figure 2.
Frequency distribution of the shell lengths (mm) of Manila clams in the sediment (solid line; n=859) and consumed by oystercatchers (dashed line; n=77). Both frequency distributions have been expressed as percentages of the sample size to facilitate comparison.
Figure 3.
An overview of published studies of the intake rates (mg AFDM s−1) of free-living, over-wintering European oystercatchers consuming native bivalve species: M. edulis (black); C. edule (open); S. plana (dark grey); and M. balthica (pale grey). Frequencies refer to the number of independent values of intake rate presented in the source paper (Zwarts et al. 1996a). The arrow denotes the value of the mean intake rate achieved by birds specializing on Manila clams (n=27) in this study (2.02 mg AFDM s−1 (95% CI 1.673–2.367 mg AFDM s−1)).
(b) Simulation modelling
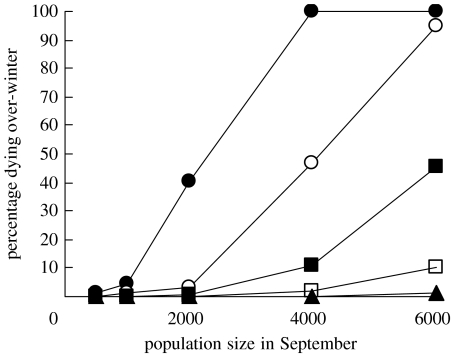
The model predicts that the presence of Manila clams in Poole Harbour, even at the current mean density of approximately 5 clams m−2 (Jensen et al. 2004), has reduced the over-winter mortality of the existing wintering population of oystercatchers (from 4.6% to 1.1%) and altered the shape of the density-dependent mortality function (figure 4). The model predicts that if the average clam abundance across Poole Harbour was to increase, the density dependence of the over-winter mortality of oystercatchers would gradually diminish in strength. The density dependence of over-winter mortality would be completely suppressed if an average density of 40 clams m−2 were reached, even were the local wintering population of oystercatchers to increase fivefold (figure 4).
Figure 4.
Variation in the form of the density-dependent mortality function of oystercatchers given various population densities of Manila clams (0 clams m−2, filled circles; 5 clams m−2, open circles; 10 clams m−2, filled squares; 20 clams m−2, open squares; 40 clams m−2, filled triangles). Each data point depicts the mean predicted over-winter mortality arising from 10 replicate simulations of each scenario. The variation between replicate simulations was so small that the 95% CIs cannot be presented given the size of the symbols.
4. Discussion
Manila clams are eaten by a variety of birds, e.g. gulls (Family Laridae), crows (Family Corvidae) and scoters (Family Anatidae) (Toba et al. 1992). However, no instance of oystercatchers eating Manila clams has ever been recorded in the scientific literature. Worldwide enquiries confirm that there is no knowledge of this predator–prey interaction. Thus, our field observations provide the first recorded instance of oystercatchers exploiting a wild population of Manila clams anywhere in the world.
Most mortality in wintering wader populations is caused by competition for limited resources leading to starvation or to risk-prone behaviour motivated by hunger (Goss-Custard 1985; Whitfield 2003) and occurs during the coldest period of the winter, usually after mid-January (Zwarts et al. 1996b). The proportion of oystercatchers consuming Manila clams reached 40–50% during this key stage of the winter. As only birds that were clearly seen to consume a Manila clam during a 10 min period were included in deriving this figure, it is a very conservative estimate. Thus, this apparently anecdotal observation of a novel predator–prey interaction could have considerable biological significance. Our behaviour-based IBM of shorebird foraging supports this assertion.
Our model indicates that the invasion of Poole Harbour by Manila clams has potentially already altered the over-winter mortality of oystercatchers there. Manila clams, however, were only introduced to the site less than 20 years ago and the current average density is comparatively low (Humphreys et al. 2007). However, the clams grow comparatively well and regularly exhibit two recruitment events per year (Humphreys et al. 2007). Densities of 60 clams m−2 already occur in some locations within the harbour (Jensen et al. 2007). Given that a density of 1000 m−2 is considered moderate in the clams' native range (Ohba 1959) and that the annual variation in water temperature within Poole Harbour (3–27°C) is similar to that in southern Brittany (Golfe du Morbihan), the lagoons of the Adriatic Sea and in the Inland Sea of Japan where the Manila clam thrives at such high densities, it would be surprising if this local population does not continue to grow to attain average densities similar to those that we have modelled.
The magnitude of the effect of an alteration to the density dependence of over-winter mortality on the size of the overall population depends upon the ratio of the strengths of density dependence of mortality in the winter and of reproductive output in the summer (Goss-Custard & Sutherland 1997). However, even quite small changes to over-winter mortality rates, whether density dependent or not, can lead to pronounced changes in population size, especially when the density dependence of reproductive success is weak (Goss-Custard 1993). Given that the population of oystercatchers in Poole Harbour comprises approximately 0.5% of the UK wintering population, it is however not surprising that the invasion of Poole Harbour by Manila clams has had no discernible effect on the size of the UK wintering population of oystercatchers, which has declined since the early 1990s (Collier et al. 2005). In fact, numbers of many species of shorebirds have been stable or have declined on the south west coasts of Britain while increasing on the east coast (Austin & Rehfisch 2005).This pattern has been explained as a large-scale population response to warming winter climate (Austin & Rehfisch 2005). This has clearly overridden any local effects of clams' presence in Poole Harbour where the size of the local wintering oystercatcher population has, in spite of the new food resources available, also not increased (Pickess & Underhill-Day 2002). Stark et al. (1999) recorded a fourfold increase in the waterbird population of Lake Constance in response to the invasion of the zebra mussel, making the site one of the most important wintering sites for waterbirds in Central Europe. It may be that the intensive winter fishery for the Manila clam in Poole Harbour which reduces the abundance, maximum age and size of Manila clams there (Humphreys et al. 2007) has suppressed the potential benefits to oystercatchers which would otherwise have already taken place.
One of the most common reasons for the reproduction and successful invasion of non-native species in marine environments is elevated seawater temperatures in relation to regional or local conditions (Eno et al. 1997). Food availability and water temperature are the principal environmental variables that control the growth, reproduction and survival of Manila clams (Bodoy et al. 1980; Maître-Allain 1982; Melià et al. 2004). The optimum water temperature for growth is between 20 and 25°C and spawning occurs when water temperatures are between 18 and 26°C (Solidoro et al. 2003). The naturalization of Manila clams in Poole Harbour, as in the lagoons of the Adriatic Sea, reflects the presence of relatively warm eutrophic waters (Jensen et al. 2004). Manila clams spawn more frequently and over longer periods in the southern part of their native range and within Europe (Laruelle et al. 1994). At present, the population of Manila clams in Poole Harbour, like those in Brittany, is subject to occasional mortality events in late winter, often associated with cold weather and negative energy budgets (Goulletquer et al. 1989; Humphreys et al. 2007). Thus, in northern European waters, the reproduction and survival of Manila clams are vulnerable in many locations under existing environmental conditions. However, increases in seawater temperature that have already occurred in European coastal waters and which are predicted to continue (Hulme et al. 2002) will favour both the reproduction and survival of Manila clams at many more sites than they currently occupy. Biological responses in the ocean to climate change will be substantially more complex than a simple response to temperature alone (Harley et al. 2006) and predicting species' distributions will therefore be equally complex (Guisan & Thuiller 2005). Nonetheless, the rate at which the biogeographic limits of southern intertidal species are extending northwards and eastwards towards the colder North Sea is up to 50 km per decade (Mieszkowska et al. 2006). Indeed, a recent survey has shown that Manila clams are now abundant on the intertidal mudflats of Southampton Water which is 50 km east of Poole Harbour. This spread may not have been natural, but mediated by man. Nonetheless, given the precedents set by several southern, warm-water species, it would not be surprising if, under future climate change scenarios, the Manila clams were to (be) spread and establish populations around the increasingly warm coastal waters of northwest Europe.
The Manila clam has only recently naturalized in European waters (Goulletquer 1997; Goulletquer & Héral 1997). Thus, it is not yet possible to be sure of the long-term consequences that its invasion may have on invaded ecosystems. When cultivated at very high densities, Manila clams are known to alter biogeochemical cycles, the abundance of microplankton, zooplankton and macroalgal growth (Sorokin et al. 1999; Bartoli et al. 2001). Manila clams may also carry diseases that are transmissible to other species (Figueras et al. 1996), compete for resources with other species and may provide a new food resource for generalist predators (Toba et al. 1992). In Venice Lagoon, the Manila clam has apparently replaced Cerastoderma glaucum and Tapes decussatus (Occhipinti-Ambrogi 2000 although see Breber 2002). Surveys of the macroinvertebrate fauna on the intertidal flats of Poole Harbour in the late 1980s and in 2002 reveal that the appearance of the Manila clam in Poole Harbour coincided with a decline in the abundance of Scrobicularia plana and M. balthica (Caldow et al. 2005). However, the decline of these species may have been caused by tri-butyl tin pollution (Langston et al. 2003) and may have facilitated the naturalization of the Manila clam. The average numerical density of bivalves as a group is more or less the same now as in the 1980s, but it is C. edule and Abra tenuis that have increased most in the intervening years rather than the Manila clam (Caldow et al. 2005). Thus, within Poole Harbour, there is no evidence yet of species replacement by the Manila clam. Within the scientific literature, there is also no evidence that at the densities typical of wild populations of Manila clams, they have any negative effects on native shellfish fauna (Breber 2002; Byers 2005). However, Mieszkowska et al. (2006) suggested that the spread of southern warm water species may compound the retreat of northern cold water species on which shorebirds may currently depend.
The ability of birds to winter further north than they currently do under future climate change scenarios is likely to be constrained by a lack of daylight and by the lack of extensive intertidal mudflats. Thus if, in response to the warming of European coastal waters, the Manila clam spreads from its current loci, this is likely to be into locations that will hold substantial populations of over-wintering shorebirds in the future. There are several species of shorebird that winter on western European coasts and regularly prey upon bivalves, i.e. oystercatcher, curlew black-tailed godwit, bar-tailed godwit Limosa lapponica, knot Calidris canutus and grey plover Pluvialis squatarola. Thus, given the probable contraction of the southern range edges of many northern species of shellfish in response to climate warming (Mieszkowska et al. 2006), we suggest that if the Manila calm were to spread, this would have the potential to be of considerable benefit to several European shorebird populations. The potential implications of our findings highlight the need for further studies to improve our understanding of the interactions between native and non-native marine species. Furthermore, given the rate of climate change and of species responses to it, there is a pressing need for studies that improve our understanding of the interactions between the drivers of biodiversity change (Sala et al. 2000) such as biotic exchange and climate change.
Acknowledgments
Thanks are due to P. Bocher, G. Burnell, R. Butler, K. Chew, J. Cigarria, P. Goulletquer, A. Perez-Hurtado de Mendoza, S. Ponurovskii, T. Piersma, N. Selin, R. Ydenberg and L. Zwarts for replying to enquiries concerning oystercatcher/clam interactions and to J. Bullock, P. Goulletquer, R. Gozlan, C. Reading, K. Schonrogge and J. Thomas for providing comments. Various parts of this work were funded by English Nature and Associated British Ports.
References
- Austin G.E, Rehfisch M.M. Shifting nonbreeding distributions of migratory fauna in relation to climate change. Glob. Change Biol. 2005;11:31–38. doi:10.1111/j.1529-8817.2003.00876.x [Google Scholar]
- Bartoli M, Nizzoli D, Viaroli P, Turolla E, Castaldelli G, Fano E.A, Rossi R. Impact of Tapes philippinarum farming on nutrient dynamics and benthic respiration in the Sacca di Goro. Hydrobiologia. 2001;455:203–212. doi:10.1023/A:1011910422400 [Google Scholar]
- Beukema J.J. Expected changes in the Wadden Sea benthos in a warmer world—lessons from periods with mild winters. Neth. J. Sea Res. 1992;30:73–79. doi:10.1016/0077-7579(92)90047-I [Google Scholar]
- Bodoy A, Maitre-Allain T, Riva A. Croissance comparée de la palourde européenne (Rudiatapes decussatus) et de la palourde japonaise (Ruditapes philippinarum) dans un écosystème artificial méditerranéen. Vie Marine. 1980;2:39–51. [Google Scholar]
- Breber P. Introduction and acclimatisation of the Pacific carpet clam, Tapes philippinarum, to Italian waters. In: Leppäkoski E, Gollasch S, Olenin S, editors. Invasive aquatic species of Europe. Distribution, impacts and management. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 120–126. [Google Scholar]
- Byers J.E. Marine reserves enhance abundance but not competitive impacts of a harvested nonindigenous species. Ecology. 2005;86:487–500. [Google Scholar]
- Caldow R, McGrorty S, West A, Durell S.E.A. le V. dit, Stillman R, Anderson S. Macro-invertebrate fauna in the intertidal mudflats. In: Humphreys J, May V, editors. The ecology of Poole Harbour. Elsevier B.V.; Amsterdam, The Netherlands: 2005. pp. 91–108. [Google Scholar]
- Collier M.P, Banks A.N, Austin G.E, Girling T, Hearn R.D, Musgrove A.J. BTO/WWT/RSPB/JNCC; Thetford, UK: 2005. The wetland bird survey 2003/2004: wildfowl and wader counts. [Google Scholar]
- Dare P.J, Bell M.C, Walker P, Bannister R.C.A. Centre for Environment, Fisheries and Aquaculture Science; Lowestoft, UK: 2004. Historical status of cockle and mussel stocks in The Wash. [Google Scholar]
- Durell S.E.A. le V. dit, Stillman R.A, Caldow R.W.G, McGrorty S, West A.D, Humphreys J. Modelling the effect of environmental change on shorebirds: a case study on Poole Harbour, U.K. Biol. Conserv. 2006;131:459–473. doi:10.1016/j.biocon.2006.02.022 [Google Scholar]
- Eno N.C, Clark R.A, Sanderson W.G. Joint Nature Conservation Committee; Peterborough, UK: 1997. Non-native marine species in British waters: a review and directory. [Google Scholar]
- Figueras A, Robledo J.A.F, Novoa B. Brown ring disease and parasites in claims (Ruditapes decussatus and R. philippinarum) from Spain and Portugal. J. Shellfish Res. 1996;15:363–368. [Google Scholar]
- Goss-Custard J.D. Foraging behaviour of wading birds and the carrying capacity of estuaries. In: Sibly R.M, Smith R.H, editors. Behavioural ecology: ecological consequences of adaptive behaviour. Blackwell Scientific Publications; Oxford, UK: 1985. pp. 169–188. [Google Scholar]
- Goss-Custard J.D. The effect of migration and scale on the study of bird populations: 1991 Witherby Lecture. Bird Study. 1993;40:81–96. [Google Scholar]
- Goss-Custard, J. D. & Stillman, R. A. In press. Individual-based models and the management of shorebird populations. Nat. Res. Model
- Goss-Custard J.D, Sutherland W.J. Individual behaviour, populations and conservation. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. An evolutionary approach. Blackwell Science Ltd; Oxford, UK: 1997. pp. 373–395. [Google Scholar]
- Goss-Custard J.D, Cayford J.T, Boates J.S, Durell S.E.A. le V. dit. Field tests of the accuracy of estimating prey size from bill length in oystercatchers Haematopus ostralegus eating mussels, Mytilus edulis. Anim. Behav. 1987;35:1078–1083. doi:10.1016/S0003-3472(87)80165-3 [Google Scholar]
- Goulletquer, P. 1997 A bibliography of the Manila clam Tapes philippinarum RIDRV-97.02/RA. La Tremblade, France: Institute française pour recherche de l'exlpoitation de la mer.
- Goulletquer, P. & Héral, M. 1997 History, present conditions and future of the molluscan fisheries of North America and Europe. Marine molluscan production trends in France: from fisheries to aquaculture. Marine Fisheries Review, NOAA Technical Report NMFS. 129, 137–164
- Goulletquer P, Héral M, Deslous-Paoli J.M, Prou J, Garnier D.R, Boromthanarat W. Ecophysiologie et bilan énergétique de la palourde japonaise d'élevage Ruditapes philippinarum. J. Exp. Mar. Biol. 1989;132:85–108. doi:10.1016/0022-0981(89)90217-7 [Google Scholar]
- Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. doi:10.1111/j.1461-0248.2005.00792.x [DOI] [PubMed] [Google Scholar]
- Hamilton D.J, Ankney C.D, Bailey R.C. Predation of zebra mussels by diving ducks: an exclosure study. Ecology. 1994;75:521–531. doi:10.2307/1939555 [Google Scholar]
- Harley C.D.G, Hughes A.R, Hultgren K.M, Miner B.G, Sorte C.J.B, Thornber C.S, Rodriguez L.F, Tomanek L, Williams S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. doi:10.1111/j.1461-0248.2005.00871.x [DOI] [PubMed] [Google Scholar]
- Hulme, M., Turnpenny, J. & Jenkins, G. 2002 Climate change scenarios for the United Kingdom: the UKCIP02 Scientific Report Norwich, UK: Tyndall Centre for Climate Change Research, School of Environmental Sciences, University of East Anglia.
- Humphreys J, Caldow R.W.G, McGrorty S, West A.D, Jensen A.C. Population dynamics of naturalised Manila clams Ruditapes philippinarum in British coastal waters. Mar. Biol. 2007 doi:10.1007/s00227-007-0660-x [Google Scholar]
- Jensen A.C, Humphreys J, Caldow R.W.G, Grisley C, Dyrynda P.E.J. Naturalization of the Manila clam (Tapes philippinarum), an alien species, and establishment of a clam fishery within Poole Harbour, Dorset. J. Mar. Biol. Assoc. UK. 2004;84:1069–1073. doi:10.1017/S0025315404010446h [Google Scholar]
- Jensen A, Humphreys J, Caldow R, Cesar C. The Manila clam in Poole Harbour. In: Humphreys J, May V, editors. The ecology of Poole Harbour. Elsevier B.V.; Amsterdam, The Netherlands: 2005. pp. 163–173. [Google Scholar]
- Kendall M.A, Burrows M.T, Southward A.J, Hawkins S.J. Predicting the effects of marine climate change on the invertebrate prey of the birds of rocky shores. Ibis. 2004;146(Suppl. 1):40–47. doi:10.1111/j.1474-919X.2004.00326.x [Google Scholar]
- Laing I.S.D, Utting S.D. The physiology and biochemistry of diploid and triploid clams (Tapes philippinarum) larvae and juveniles. J. Exp. Mar. Biol. Ecol. 1994;184:159–169. doi:10.1016/0022-0981(94)90002-7 [Google Scholar]
- Langston W.J, Chesman B.S, Burt G.R, Hawkins S.J, Readman J, Worsfold P. Marine Biological Association; Plymouth, UK: 2003. Site characterisation of the south west European marine sites. Poole Harbour SPA. Marine Biological Association Occasional Publication No 12. [Google Scholar]
- Laruelle F, Guillou J, Paulet Y.M. Reproductive pattern of the clams, Ruditapes decussatus and R. philippinarum on intertidal flats in Brittany. J. Mar. Biol. Assoc. UK. 1994;74:351–366. [Google Scholar]
- Lawrence A.J, Soame J.M. The effects of climate change on the reproduction of coastal invertebrates. Ibis. 2004;146(Suppl. 1):29–39. doi:10.1111/j.1474-919X.2004.00325.x [Google Scholar]
- Leopold, M. F., Spannenburg, P. C., Verdaat, H. J. P. & Kats, R. K. H. In press. Identification and size estimation of Spisula subtruncata and Ensis americanus from shell fragments in stomachs and faeces of common eiders Somateria mollissima and common scoters Melanitta nigra Atlantic Seabirds
- Leppäkoski E, Gollasch S, Olenin S. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. Invasive aquatic species of Europe. Distribution, impacts and management. [Google Scholar]
- Maître-Allain T. Influence du milieu sur la croissance de deux palourdes, Ruditapes decussatus et Ruditapes philippinarum, dans l'étang de Thau (Hérault) Vie Marine. 1982;4:37–50. [Google Scholar]
- Melià P, De Leo G.A, Gatto M. Density and temperature-dependence of vital rates in the Manila clam Tapes philippinarum: a stochastic demographic model. Mar. Ecol. Prog. Ser. 2004;272:153–164. [Google Scholar]
- Mieszkowska N, Kendall M.A, Hawkins S.J, Leaper R, Williamson P, Hardman-Mountford N.J, Southward A.J. Changes in the range of some common rocky shore species in Britain—a response to climate change? Hydrobiologia. 2006;555:241–251. doi:10.1007/s10750-005-1120-6 [Google Scholar]
- Mooney H.A, Cleland E.E. The evolutionary impact of invasive species. Proc. Natl Acad. Sci. USA. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. doi:10.1073/pnas.091093398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhipinti-Ambrogi A. Biotic invasions in a Mediterranean lagoon. Biol. Invasions. 2000;2:165–176. doi:10.1023/A:1010004926405 [Google Scholar]
- Occipinti-Ambrogi A, Savini D. Biological invasions as a component of global change in stressed marine environments. Mar. Poll. Bull. 2003;46:542–551. doi: 10.1016/S0025-326X(02)00363-6. doi:10.1016/S0025-326X(02)00363-6 [DOI] [PubMed] [Google Scholar]
- Ohba S. Ecological studies in the natural population of a clam, Tapes japonica, with special reference to seasonal variations in the size and structure of the population and to individual growth. Biol. J. Okayama Univ. 1959;5:13–43. [Google Scholar]
- Pickess B.P, Underhill-Day J.C. Poole Harbour Study Group; Wareham, UK: 2002. Important birds of Poole Harbour. [Google Scholar]
- Richman S.E, Lovvorn J.R. Relative foraging value to lesser scaup ducks of native and exotic clams from San Francisco Bay. Ecol. Appl. 2004;14:1217–1231. [Google Scholar]
- Sala O.E, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. doi:10.1126/science.287.5459.1770 [DOI] [PubMed] [Google Scholar]
- Smit C, Piersma T. Numbers, mid-winter distribution and migration of wader populations using the East Atlantic flyway. In: Boyd H, Pirot J.-Y, editors. Flyways and reserve networks for waterbirds. International Wildfowl and Wetlands Research Bureau; Slimbridge, UK: 1989. pp. 24–63. [Google Scholar]
- Solidoro C, Melaku Canu D, Rossi R. Ecological and economic considerations on fishing and rearing of Tapes philippinarum in the lagoon of Venice. Ecol. Model. 2003;170:303–318. doi:10.1016/S0304-3800(03)00235-7 [Google Scholar]
- Sorokin I.I, Giovanardi O, Pranovi F, Sorokin P.I. Need for restricting bivalve culture in the southern basin of the lagoon of Venice. Hydrobiologia. 1999;400:141–148. doi:10.1023/A:1003707231839 [Google Scholar]
- Stark, H., Bauer, H.-G., Suter, W. & Jacoby, H. 1999 Internationale Wasservogelzählung am Bodensee. Ergebnisse aus den Zählperioden 1961/62 bis 1996/97. Dynamik der Zugrast- und Überwinterungbestände und der Einfluß von Umweltbedingungen. In Die Vögel des Bodenseegebietes (eds G. Heine, H. Jacoby, H. Leuzinger & H. Stark), pp. 64–122.
- Suter W. Der Einfluss von Wasservögeln auf Populationen der Wandermuschel (Dreissena polymorpha Pall.) am Untersee-Ende/Hochrhein (Bodensee) Schweizerische Zeitschrift für Hydrologie. 1982a;44:149–161. doi:10.1007/BF02502194 [Google Scholar]
- Suter W. Die Bedeutung vom Untersee-Ende/Hochrhein (Bodensee) als wichtiges Überwinterungs-gewässer für Tauchenten (Aythya, Bucephala) und Bläßhuhn (Fulica atra) Ornithologischer Beobachter. 1982b;79:73–96. [Google Scholar]
- Toba D.R, Thompson D.S, Chew K.K, Anderson G.J, Miller M.B. Washington Sea Grant Publication, University of Washington; Seattle, WA: 1992. Guide to Manila clam culture in Washington. [Google Scholar]
- Werner S, Mörtl M, Bauer H.-G, Rothhaupt K.-O. Strong impact of wintering waterbirds on zebra mussel (Dreissena polymorpha) populations at lake Constance, Germany. Freshw. Biol. 2005;50:1412–1426. doi:10.1111/j.1365-2427.2005.01411.x [Google Scholar]
- West A.D, Goss-Custard J.D, McGrorty S, Stillman R.A, Durell S.E.A. le V. dit, Stewart B, Walker P, Palmer D.W, Coates P.J. The Burry shellfishery and oystercatchers: using a behaviour-based model to advise on shellfishery management policy. Mar. Ecol. Prog. Ser. 2003;248:279–292. [Google Scholar]
- Whitfield D.P. Predation by Eurasian sparrowhawks produces density-dependent mortality of wintering redshanks. J. Anim. Ecol. 2003;72:27–35. doi:10.1046/j.1365-2656.2003.00672.x [Google Scholar]
- Wormington A, Leach J.H. Concentrations of migrant diving ducks at Point Pelee, Ontario, in response to invasion of zebra mussels, Dreissena polymorpha. Can. F. Nat. 1992;106:376–380. [Google Scholar]
- Zwarts L, Ens B.J, Goss-Custard J.D, Hulscher J.B, Durell S.E.A. le V. dit. Causes of variation in prey profitability and its consequences for the intake rate of the oystercatcher Haematopus ostralegus. Ardea. 1996a;84A:229–268. [Google Scholar]
- Zwarts L, Hulscher J.B, Koopman K, Piersma T, Zegers P.M. Seasonal and annual variation in body weight, nutrient stores and mortality of oystercatchers Haematopus ostralegus. Ardea. 1996b;84A:327–356. [Google Scholar]