Abstract
We review a program of research that uses neuroimaging techniques to determine the functional and neural architecture of human working memory. A first set of studies indicates that verbal working memory includes a storage component, which is implemented neurally by areas in the left-hemisphere posterior parietal cortex, and a subvocal rehearsal component, which is implemented by left-hemisphere speech areas, including Broca’s area as well as the premotor and supplementary motor areas. We provide a number of neuroimaging dissociations between the storage and rehearsal areas. A second set of studies focuses on spatial working memory and indicates that it is mediated by a network of predominantly right-hemisphere regions that include areas in posterior parietal, occipital, and frontal cortex. We provide some suggestive evidence that these areas, too, divide into storage and rehearsal regions, with right-hemisphere posterior parietal and premotor regions subserving spatial rehearsal. In a final set of studies, we turn to “executive processes,” metaprocesses that regulate the processing of working-memory contents. We focus on the executive process of inhibition as it is used in verbal working memory. We provide evidence that such inhibition is mediated by the left-hemisphere prefrontal region and that it can be dissociated from verbal storage and rehearsal processes.
Working memory (WM) allows humans (and other species) to maintain a limited amount of information in an active state for a brief period of time and to manipulate that information (1). Typically, the amount of information kept active, or “on-line,” ranges between 1–10 items whereas the duration of that storage ranges from 0–60 seconds. A paradigmatic case of WM used solely for maintenance would be storing a phone number until you dial it. A paradigmatic case of WM used for manipulation as well as maintenance would be constructing a “mental map” of an area while someone gives you directions on how to find a particular house there. It is the latter kind of case that has made WM a topic of intense interest to psychologists, as the on-line manipulation of material may be a cornerstone of higher cognitive processes, such as reasoning, decision-making, problem solving, and language understanding (e.g., refs. 2 and 3).
WM is not a unitary system. Rather, there appear to be different WMs for different kinds of information. In particular, behavioral, neuropsychological, and neuroimaging evidence converge in indicating that there are separate systems for verbal and spatial information (4). Some neurological patients are impaired in WM tasks for verbal information but perform relatively normally on WM tasks for spatial information whereas other patients show the reverse pattern; and, in neuroimaging studies, different cortical regions typically are activated in verbal and spatial WM tasks. Accordingly, in this article we treat the two systems separately.
The line of research that we summarize in this article has been concerned with the use of neuroimaging to determine the basic architecture of verbal and spatial WM. To preview the discussion, our research suggests that each system has three different functional components, with distinct neural implementations: (i) a pure storage component, whose contents decay rapidly; (ii) a rehearsal component that can reactivate the rapidly decaying contents of the storage component; and (iii) an “executive” component that regulates the processing of the contents of WM (unlike the preceding components, executive processes may be the same for verbal and spatial WM). Situations that require people just to maintain information for a brief time period (such as the telephone number example) require only the storage and rehearsal components whereas situations that require people to manipulate information that is being maintained in WM (such as the mental map example) often involve the executive component as well.
In the following discussion, we begin with neuroimaging studies of verbal WM in situations that emphasize the maintenance of information. These experiments provide evidence for both a short-term phonological store and a subvocal rehearsal process. Then we consider comparable neuroimaging studies of spatial WM when this system is used primarily for maintenance; these studies point to the existence of a short-term spatial store and a spatial rehearsal process. In the last section, we consider tasks that require the use of the executive component as well as storage and rehearsal components.
All of the experiments that we review used positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) as imaging modalities. These experiments produced significant deactivations as well as activations, but, because the interpretation of deactivations remains problematic at this point in time, we focus on the activations (see ref. 5 for a discussion of deactivations in general; see ref. 6 for a discussion of deactivations in a WM study).
Storage and Rehearsal Processes in Verbal WM
Neuroimaging Experiments.
Our neuroimaging studies took as a starting point a view of WM that was derived from cognitive–behavioral studies from the mid-1960s through the early 1990s. One influential summary of that literature was Baddeley’s (e.g., refs. 1 and 7) proposed architecture of WM. It consisted of a “central executive” (what we here call “executive processes”) and two storage devices corresponding to what we have termed “verbal WM” and “spatial WM.” Baddeley further maintained that verbal WM could be decomposed into a “phonological buffer” that allows for the short-term maintenance of phonological information and a rehearsal process that refreshes the contents of the buffer. Our first neuroimaging experiment with verbal materials produced results in line with Baddeley’s decomposition of verbal WM. (This experiment, like all others described in this article, involved visual presentation.)
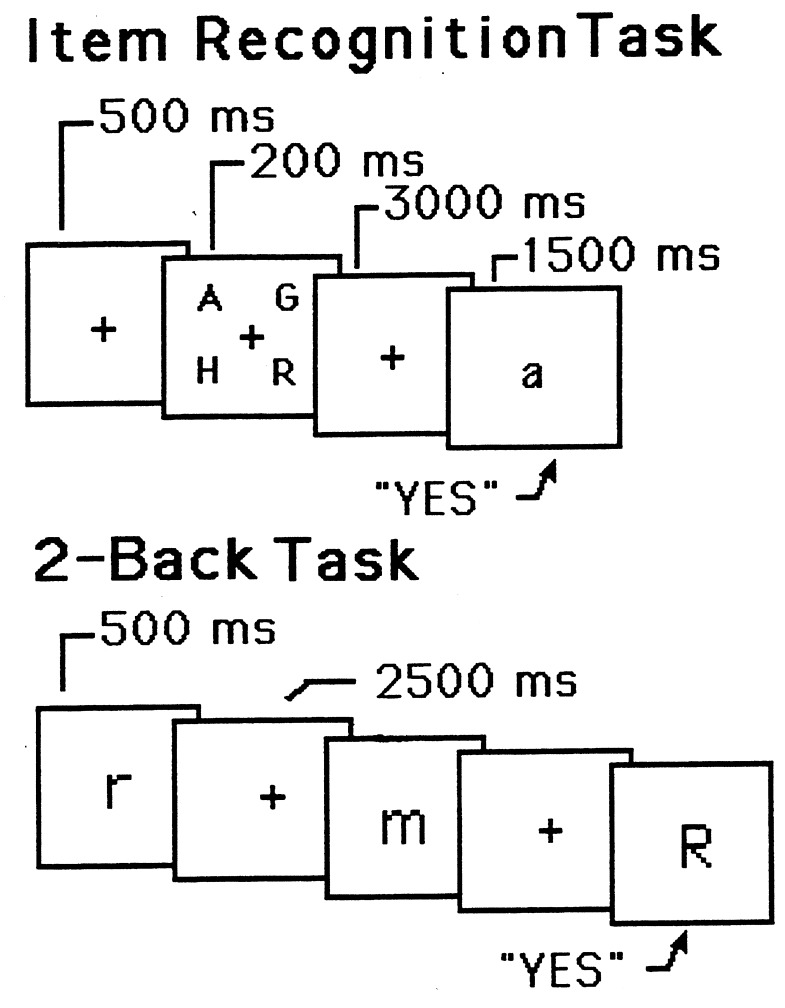
In the experiment (8), subjects were PET-imaged while they performed an item-recognition task (9). The task is presented schematically in the top of Fig. 1. On each of a series of discrete trials, four target letters were presented simultaneously, followed by a 3,000-ms blank delay period (during which subjects had to remember the letters), followed by a probe letter, to which subjects responded “yes” or “no” (by pressing one of two buttons) indicating whether the probe was identical in name to one of the four target letters. This task necessitates verbal WM, but it includes other processes as well—e.g., perception of the letters and execution of a response—and, consequently, the PET image acquired during performance of the task included activations caused by the additional processes as well as activations caused by WM. To eliminate the unwanted activations, subjects also participated in a control task, which presumably contained the unwanted processes but not the WM ones. The task was similar to the memory task except that the probe was presented immediately after the target letters (there was no delay), and the latter remained in view along with the probe. Hence, no memory was needed to accomplish the control task.
Figure 1.
Schematic representations of trials in two different WM tasks. (Upper) A sample trial for the item-recognition task. It includes the following: (i) a fixation point, (ii) four uppercase letters, (iii) a blank delay interval, and (iv) a lowercase probe letter. The subject’s task is to decide whether the probe names one of the four target letters. (Lower) A sample trial for the 2-back task. Each letter is followed by a blank delay interval. The subject’s task is to decide whether each letter has the same name as the one that occurred two back in the sequence. The durations for all trial events are shown. Adapted from Smith et al. (38).
When the image acquired during the control task was subtracted from that acquired during the memory task, there were several activations, mostly in the left hemisphere. These included: left posterior parietal cortex (Brodmann Area, or BA 40), Broca’s area (BA 44), left premotor area (BA 6), and left supplementary motor area (BA 6). Given that the latter three areas are known to be involved in the planning and production of speech (e.g., 10), we tentatively identified them as mediating a subvocal, rehearsal process; the posterior parietal area was thought to underlie a storage process. (Unless otherwise noted, whenever we discuss these four areas in the context of verbal WM, we are referring to left-hemisphere regions).
There is stronger evidence for a dissociation between storage and rehearsal processes in another PET experiment reported by Awh and colleagues (8). This study used a “2-back” paradigm (e.g., ref. 11), which is presented schematically in the bottom of Fig. 1. Subjects viewed a continuous stream of letters, each presented for 500 ms, with a 2,500-ms interval between successive letters. For each letter, subjects had to decide whether it was identical in name to the letter two back in the sequence. Two different control conditions were used. One was a search task: Subjects saw the same kind of sequence of letters as in the memory condition but now simply decided whether each letter matched a single target letter specified at the beginning of the experiment. This control should involve perceptual and response processes identical to those in the 2-back condition, and, so, subtracting the control from the 2-back condition should yield activations in the areas responsible for WM, areas presumably similar to those obtained in the item-recognition task. This was the case, as the subtraction image showed activations in the frontal speech regions and posterior parietal cortex.
The second control condition was intended to subtract out rehearsal as well as perception and response. In this rehearsal control, subjects pressed a button when a new letter appeared, silently rehearsed it until the next letter appeared, pressed a button and rehearsed the new letter, and so on. Subtracting this control from the 2-back memory condition should have removed much of the rehearsal circuit. Indeed, in this subtraction image, neither Broca’s area nor the premotor area was significantly active. The results provide evidence that a rehearsal component can be isolated from the rest of the neural circuitry for verbal WM.
Further evidence for this claim comes from a direct comparison of the two control conditions (which we have not reported previously). According to the logic of the experiment, the search control involves “a bit” of storage and possibly “a bit” of rehearsal (because one item, the target, must be maintained) whereas the rehearsal control involves substantial rehearsal but very little storage. It follows, then, that subtracting the search control from the rehearsal control should reveal some of the rehearsal circuitry. This subtraction, in fact, revealed activation in supplementary motor area and Broca’s area. By similar reasoning, the opposite subtraction—search minus rehearsal—should reveal some activation in the memory area; indeed, this subtraction yielded activation in the posterior parietal region.
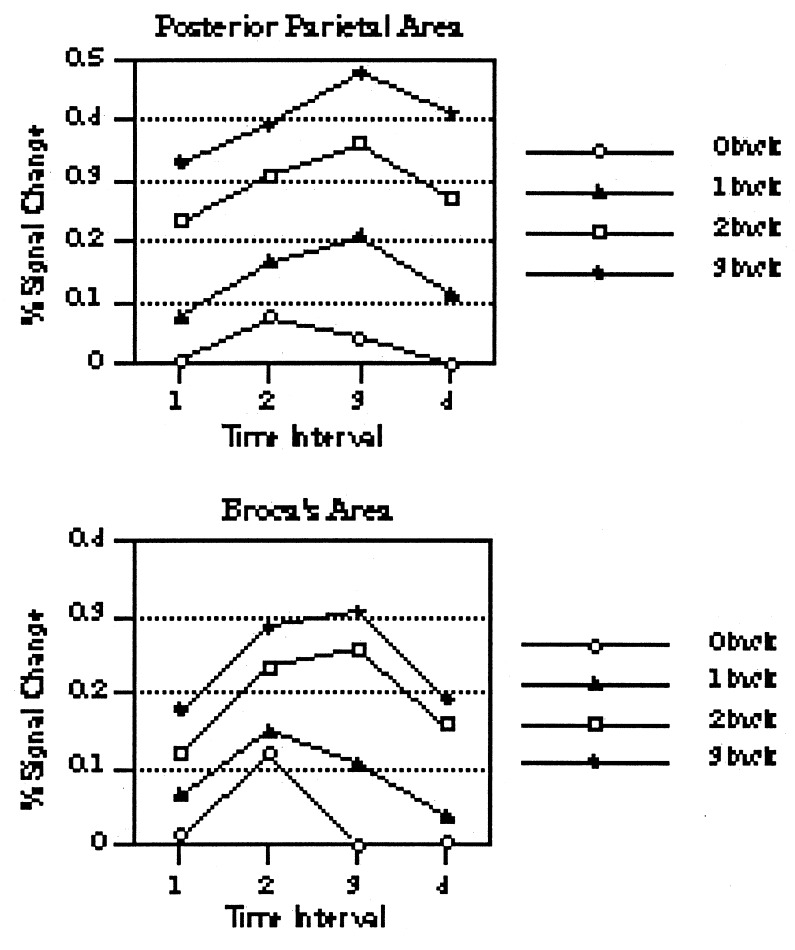
Our next source of evidence for a dissociation between storage and rehearsal came from a somewhat different kind of experiment (12). There were two major changes from the prior 2-back study. First, rather than relying on a comparison of a memory and a control condition, we varied memory load incrementally across a set of conditions. We used the n-back task—though with the interval between successive letters increased to 10,000 ms—and we varied whether subjects had to respond to memory matches 0-, 1-, 2-, or 3-back from the current item. In the 0-back task, subjects had to decide whether each letter matched a fixed target letter specified at the beginning of the letter sequence (the search task of our previous experiment); in the 1-back task, subjects had to decide whether each letter matched the one 1-back in the sequence; and so on for 2- and 3-back. If a neural area is involved in WM, its activation should increase monotonically with memory load. The second major change from the previous study was that we used fMRI rather than PET as the imaging modality. Using echoplanar imaging, we were able to scan subjects performing the tasks at four time periods during the 10-sec delay period: 0–2.5 seconds, 2.5–5.0 seconds; 5.0–7.5 seconds; and 7.5–10.0 seconds. This allowed us to determine the temporal dynamics of areas that were sensitive to WM load so that we could see whether the temporal pattern of activation differed for the posterior parietal area that presumably mediates storage versus an area that presumably mediates rehearsal (for this purpose, we focused on Broca’s area).
The relevant data are presented in Fig. 2, and they provide evidence for a dissociation between Broca’s area and the posterior parietal area. In general, activation is better sustained across the delay period for the parietal area than for Broca’s area. Indeed, Broca’s-based rehearsal seems not to be needed much for a memory load of one item, particularly deep into the delay period. It is also worth pointing out that the increase in activation with memory load was more linear for the parietal area than for Broca’s area. (In Fig. 2, compare the increases in activation with memory load for Broca’s versus posterior parietal at any of the last three temporal points.)
Figure 2.
Percentage of MRI signal change in activation as a function of temporal interval, with working memory load as the parameter. The results for the posterior parietal area are on the top, and the results for Broca’s area are on the bottom. Adapted from Cohen et al. (12)
Converging Evidence and a Sketch of a Model.
The preceding results indicate that Broca’s area consistently is activated in tasks involving subvocal rehearsal whereas the posterior parietal area is activated whenever short-term storage of verbal material is needed. However, these results tell us only that activations in the relevant areas co-occur with the cognitive functions of interest. To claim that activations are actually necessary conditions for the cognitive functions, we need evidence that damage to these areas impairs the corresponding functions. More specifically, if Broca’s area mediates rehearsal, then a lesion in this area should disrupt performance on all tasks requiring rehearsal; and, similarly, if the posterior parietal area mediates short-term storage, then a lesion in this area should disrupt performance on all tasks requiring such storage. Without this kind of converging evidence, claims about mapping brain regions onto cognitive functions remain tenuous at best (see ref. 13 for discussion).
Lesions in Broca’s area often give rise to “Broca’s aphasia,” one of the most common forms of language disorders. The most salient symptom of Broca’s aphasia is dysfluency in the production of speech; presumably, this global symptom reflects problems in the planning, generation, and execution of articulatory sequences (14). Planning and generation of articulatory sequences also may comprise core processes of subvocal rehearsal. Hence, a lesion in Broca’s area leads to problems in what may be core processes of subvocal rehearsal, which offers some converging evidence for the claim that Broca’s area mediates rehearsal. Specific findings about Broca’s aphasics and memory provide more direct evidence for the involvement of Broca’s area in subvocal rehearsal. In a verbal WM task with substantial delays, Broca’s aphasics show a steeper forgetting curve than do Korsakoff’s patients; in contrast, this difference in forgetting rates reverses in a visual WM task. Thus, the aphasic’s poorer performance in the verbal task is likely caused by the lack of subvocal rehearsal rather than by a general memory problem (15).
Other evidence from language disorders supports the claim that the left posterior parietal area mediates a pure storage function. In “conduction aphasia,” a common lesion cite is in the left-hemisphere posterior parietal cortex, and the most common symptom is an inability to repeat back verbal material, even when the delay is minimal so that rehearsal is not a major factor. Furthermore, this repetition deficit is specific to verbal material (conduction aphasics perform relatively normally on spatial WM tasks). This pattern of results implies a deficit in the storage component of verbal WM (e.g., ref. 16) and implies further that this component is mediated by the left posterior parietal area, which converges nicely with our neuroimaging findings.
Other findings about conduction aphasics suggest a new interpretation of the verbal storage function. In addition to their problems in remembering phonological sequences, Conduction aphasics show other phonological problems, such as transposing the order of sounds in a word or inserting extra phonemes in a word (17). These findings imply that the posterior parietal area generally is involved in phonological processing and is not restricted to a memorization function. This raises the possibility that the parietal area of interest may be activated in our neuroimaging studies because it is part of the circuitry involved in translating the visually presented verbal materials into a phonological code, and then the area simply remains active for a few seconds, where this persistence of activity constitutes the pure storage function of verbal WM. In essence, short-term verbal storage is a temporary activation of long-term memory rather than a separate buffer designed for a special purpose (e.g., ref. 18).
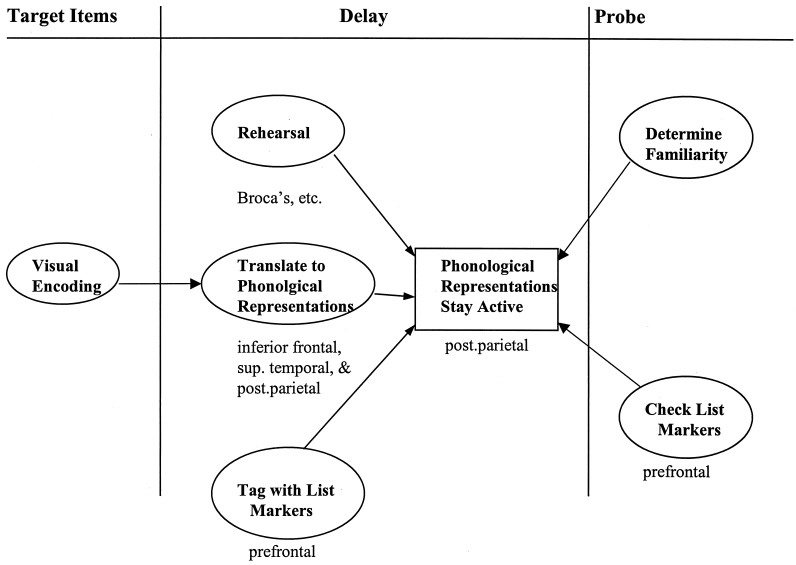
These ideas are incorporated into the schematic model of verbal WM sketched in Fig. 3. The model describes the cognitive functions involved in the item-recognition task discussed at the beginning of our review (the task events are repeated at the top of Fig. 3) and specifies the rough neural implementations of some of these processes. The bulk of the figure portrays the processes that occur during the delay period—after the letters have been identified as visual forms but before their representations have been matched to the probe.
Figure 3.
Schematic model of verbal WM. The model describes some of the cognitive functions involved in the item-recognition task and, where possible, specifies their rough neural implementations. (See text for explanation.)
Let us flesh out these delay-period processes. The subject first has to translate the visual letter representations into their corresponding phonological representations (the names of the letters). The phonological representations that are created remain active, though only for a few seconds (other than the single-item exception noted below). A subvocal rehearsal process that essentially computes the articulatory codes needed to speak the letter names can refresh these representations. We assume that the phonological translation process is mediated in part by the posterior parietal area but that other areas, including the inferior frontal cortex and superior temporal cortex, are involved as well (e.g., refs. 19 and 20). The posterior parietal area presumably mediates the storage of the phonological representations whereas the rehearsal process is mediated by the frontal speech areas, including Broca’s area. Because the rehearsal processes is itself a strategy, presumably it is initiated by decision mechanisms (not shown in Fig. 3), which are mediated by prefrontal regions; such regions would not show up in our neuroimaging studies because the decision to initiate rehearsal may be made just once, before scanning even begins.
The remaining delay-period process in Fig. 3 consists of associating, or “tagging,” each stored representation of a target letter with a marker indicating that it is a member of the current target set. In principle, such a coding process might be thought to be mediated by prefrontal regions (see the arguments advanced in the last section of this paper), but we have found no evidence of prefrontal involvement in item-recognition tasks using four (or fewer) letters as targets. It is possible, though, that, with only four targets, coding processes are not taxed sufficiently to leave a clear-cut neural signature. A recent fMRI study of the item-recognition task found significant prefrontal activation with six target letters but not with three (21).
The model’s claims about delay-period activity are compatible with the neuroimaging dissociations we have obtained between the rehearsal circuit, particularly Broca’s area, and the posterior parietal area. The fact that we were able to subtract out some of the rehearsal circuit from verbal WM activations (reported in ref. 8) fits with the claim in Fig. 3 that the rehearsal component is a separable component, neurologically as well as cognitively. The model is also compatible with the other dissociations we reported between Broca’s and the posterior parietal areas, though in no sense does it predict them. Behavioral findings (e.g., ref. 22) suggest that it is possible to keep a single item active without rehearsal (perhaps it decays at a very slow rate). Accordingly, with a load of one item, as in the 1-back task, there is little activation in Broca’s area but some in the posterior parietal area, giving rise to the results observed in our fMRI study of n-back tasks (the last point rests on a comparison of activations in different areas, which is problematic). The lack of rehearsal with a minimal memory load may become even more striking as the delay period lengthens, as indicated by the dissociation between the temporal patterns of Broca’s area and the posterior parietal area previously presented in Fig. 2.
With regard to the recognition processing that ensues when the probe appears, the model assumes that subjects make their recognition decision by considering the status of the stored representation of the probe. Subjects presumably consider the familiarity level of the stored probe representation (a probe representation corresponding to a target should be very familiar because of rehearsal), as well as whether the stored representation of the probe contains a marker indicating it is a current target, or subjects may consider both. Finding either a high-level of familiarity, or a current-target-set marker, should lead to a “yes” decision about the probe; finding neither should lead to a “no” decision (e.g., ref. 23). The representations that are interrogated by the familiarity and tagging processes likely are stored in posterior parietal cortex.
Storage and Rehearsal Processes in Spatial WM
We now turn to spatial WM. In our initial study of this system, we performed a PET experiment of the item-recognition task parallel to the one we presented at the outset of our review, except that the target items consisted of dots rather than letters (24). On each trial, three dots were presented simultaneously at randomly chosen locations, followed by a 3,000-ms blank delay period, followed by a probe circle, to which subjects responded “yes” or “no,” indicating whether the probe encircled one of the target locations. Again we used a “no-memory” control to subtract out unwanted activations caused by perception and response. The control was similar to the spatial-memory task except that the probe was presented immediately after the target locations, and the latter remained in view along with the probe.
The major difference between the current study and the earlier item-recognition experiment was whether the material was spatial or verbal; this difference dramatically altered the pattern of activations. When the image acquired during the spatial-control task was subtracted from the image acquired during the spatial-memory task, all significant activations were in the right hemisphere (in contrast to the primarily left-hemisphere activations in the verbal item-recognition task). The critical right-hemisphere activations in the spatial task included posterior parietal cortex (BA 40), anterior occipital cortex (BA 19), the premotor area (BA 6), and an inferior prefrontal site (BA 47).
We have obtained activations in roughly these same areas in other studies of spatial WM. One experiment also used dots†, but other studies used irregular forms or letters to mark the target locations. The activations in these follow-up experiments typically have been somewhat more bilateral than those described above (but never left-lateralized, as in the verbal item-recognition task). These follow-up studies also have revealed that there are two critical sites in right posterior parietal cortex, an inferior one (BA 40) and a superior one (BA 7) (see ref. 25 for a partial review). The upshot is that spatial WM seems to be mediated by a network of predominantly right-hemisphere regions that include areas in posterior parietal, occipital, and frontal cortex.
Again, there is some converging evidence from neurological patients. Patients with lesions in the right inferior posterior parietal area are impaired selectively in spatial processing and spatial memory. For example, such patients may be unable to decide whether the two segments of a bisected line are equal in length or to maintain correct spatial relations between objects when copying a drawing. Lesions in the superior posterior parietal site are more associated with impairments in spatial attention (26).
Can the activations obtained in our spatial WM tasks be divided into storage and rehearsal functions, as was done for verbal WM? To do so, first we have to explicate the notion of spatial rehearsal. Our hypothesis is that rehearsing a spatial location in WM involves selectively attending to a representation of that location (and recomputing its spatial coordinates). We have tested this claim indirectly by comparing the imaging results obtained in our studies of spatial WM with those obtained in neuroimaging experiments of selective attention. An extensive review of the latter experiments indicates that two of the regions routinely activated also are activated in our WM studies—the premotor area and the superior posterior parietal area (27). These two regions may mediate spatial rehearsal. (see also ref. 28). Two regions that are activated in our spatial WM studies that are not activated in studies of selective attention are the inferior posterior parietal area and the anterior occipital area. These regions may mediate the storage function of spatial WM. The latter two regions may be involved in initially perceiving the locations or may be adjacent to such perceptual areas; if so, the putative storage areas again may involve persistence of activation in areas that mediate the initial encoding of the material or activation in adjacent areas.
We have tested our spatial attention hypothesis more directly in both behavioral and neuroimaging experiments. The logic behind these studies is this: If a subject is attending selectively to a particular location during the delay period of a memory task (as our rehearsal hypothesis claims), then any stimulus presented in that location during the delay will benefit from the already present attention. In a behavioral study (29), subjects performed two tasks simultaneously. One task required spatial WM, as subjects had to remember the position of a single dot during a 5,000-ms delay. The other task required subjects to make a discriminative response to a nondot test item presented during the delay. The major variation was whether the test item in the discrimination task was in the same location that subjects were rehearsing in the memory task. As predicted, subjects responded faster to the discrimination test item when it appeared in the rehearsal position than when it appeared elsewhere, supporting the notions that subjects were attending selectively to this position and that selective attention is part of spatial rehearsal.
The neuroimaging experiment that we performed to test the spatial rehearsal hypothesis used fMRI (30). A dual task was again used, with two modifications: (i) The targets included three dots, all of which were presented either in the left or the right visual field; and (ii) during the delay period, a bilateral flickering grid was presented, which occluded all potential to-be-remembered positions. If spatial attention is indeed oriented to the to-be-remembered locations (as the rehearsal hypothesis claims), there should be enhanced activations in extrastriate regions of the visual cortex that are contralateral to the to-be-memorized positions. [This prediction is based on the finding that selective attention to a position in space results in increased activation in extrastriate cortex of the contralateral hemisphere (31)]. As expected, in those extrastriate areas that were responsive to stimulation by the grid, there was greater activation in the areas contralateral to the memorized locations. A control condition established that these results were caused by the subject’s rehearsal during the delay period and not by their initial perception of the lateralized targets. There is, then, relatively direct support for the hypothesis that spatial rehearsal involves selective spatial attention and indirect support for the partitioning of spatial WM into rehearsal and storage components.
Executive Processes and Verbal Working Memory
Nature of Executive Processes.
The third architectural component of a WM system consists of “executive processes.” Researchers differ in their conceptions of such processes, though most assume that the processes somehow regulate the operation of WM and are mediated by the frontal lobes. We concur with the preceding claims and, in addition, assume that executive processes are few in number and more like “metaprocesses”: i.e., operations performed on processes themselves (e.g., ref. 3).
We propose that executive processes include at least the following three operations: (i) inhibiting a response tendency or a mental process, either of which may be tied to WM processing; (ii) selectively attending to (or enhancing) a mental process that requires access to WM and switching attention between different processes (it is possible that attending and switching attention are two different processes); and (iii) coding and checking representations in WM for an aspect other than their intrinsic content: for example, coding the contents of WM by their temporal order of appearance or checking a WM representation for such temporal information. (Again, it is possible that coding and checking may be two distinct processes.) These three kinds of operations—inhibition, attention switching, and contextual coding and checking—are cited frequently as executive processes, and the first two of them have figured centrally in computational accounts of executive processing (e.g., refs. 32 and 33) or frontal-lobe function (34). Furthermore, a recent psychometric analysis of numerous tasks that reflect frontal-lobe function suggested that only three underlying factors were involved, namely, inhibition, attention-switching, and “monitoring,” where the latter often involves checking contextual markers (A. Miyake, personal communication).
All three executive processes have been tied to the prefrontal cortex. Lesions in the prefrontal cortex of humans or other primate species selectively impair performance on tasks that require (i) inhibition (35), (ii) switching between two different processes that require access to WM‡, and (iii) temporally coding the contents of WM (e.g., ref. 10). Similarly, in neuroimaging experiments, the prefrontal cortex is activated in tasks that require response competition and inhibition (36), switching attention between two subtasks that must be performed during the same time period, (37), or temporally coding items stored in WM (e.g., ref. 12).
The above studies offer a clear link between executive processes and prefrontal cortex, but they do not provide evidence that executive processes constitute a separable component from the storage and rehearsal functions of WM. In an earlier PET study (38), we tried to support such a dissociation by contrasting a WM task that seemed to involve mainly storage and rehearsal—a four-target, item-recognition task—with a WM task that required extensive temporal coding as well—the 2-back task. We found that the 2-back task activated dorsolateral prefrontal cortex whereas the item-recognition task did not, but this apparent dissociation was compromised by the fact that there were numerous other differences in activation between the two tasks. Here we review a more successful attempt to dissociate an executive process, inhibition, from storage and rehearsal processes in verbal WM (39).
Inhibition in the Item Recognition Task.
A WM task that does not seem to involve much executive processing is the verbal item-recognition task described at the beginning of our review—four target letters, followed by a 3,000-ms delay, followed by a probe. In two prior PET studies with this task, we did not obtain any indication of activation in prefrontal areas, which is a neural signature of executive processing (8, 40). We now want to contrast this basic task with a minimal modification of it that includes inhibition. To create the latter, we modified some of the negative-probes, that is, probes that were not targets and hence required “no” responses. In our modification, half of the negative probes had been targets on the previous trial (“recent negative probes”) and so, presumably, were still in an active state. Because subjects often respond positively to any probe whose representation in WM is active or familiar (as described in the model in Fig. 3), to maintain an acceptable level of accuracy, they would have to inhibit this familiarity process, or its outcome, and respond on another basis; presumably, this other basis involves checking whether the representation of the probe is marked as a target (see Fig. 3). (Note that, while we talk of “inhibition,” response competition is involved also; studies comparable to ours that will be discussed later are labeled as “response–competition” experiments).
In a PET study, we contrasted the recent negative version of the item-recognition task with the “standard” version of the task (in which a negative probe never appeared in the previous two sets of targets). These two conditions were identical except that half of the negative probes were recent ones in the recent negatives condition (thus, only a quarter of the trials differed). We also included a control condition. In the control, there was only one target letter (though it appeared four times in the display, so that the perceptual input would be comparable to the other conditions), and the delay was only 200 ms.
Fig. 4 presents the results of the PET activations superimposed on a surface rendering of a brain, separately for three subtractions: (i) the recent negatives minus control condition, (ii) the standard minus control condition, and (iii) the recent negatives minus standard condition. Relative to the control, the standard condition produced activations in the usual left-hemisphere regions, including the frontal speech regions, and the posterior parietal cortex (not visible in Fig. 4, which shows only those activations that occurred within 15 mm of the cortical surface). The recent negatives condition produced activations in these same areas (again, relative to the control), but, of importance, it also led to a significant activation in left prefrontal cortex (BA 45). This critical difference in results between the two experimental conditions is highlighted in the recent negatives minus standard subtraction (bottom row of Fig. 4); it revealed only one significant activation (after correction for multiple comparisons), which was in the left prefrontal cortex (BA 45). Thus, the recent negatives condition differs from the standard only in that it involves an inhibition process, and only the recent negatives condition activates prefrontal cortex.
Figure 4.
Images reflecting the activations in three subtractions. Each row presents left lateral, superior, and right lateral views of the brain, with t statistics of activations at or within 15 mm of the surface superimposed in color on a gray MRI of a single subject (not one from this study). The color scale shows the magnitude of the t statistic that corresponds to each color. Shown are all activations that passed a criterion of P < .05 uncorrected for multiple comparisons. The top row depicts the sites of activation subtracting the control from the recent negatives condition; the second row depicts the sites from the subtraction of the control from the standard condition; and the bottom row shows the subtraction of the standard from the recent negatives condition. Adapted from Jonides et al. (39).
These results provide some evidence for a neural dissociation between an executive process on the one hand and storage-plus-retrieval processes on the other. However, aspects of the experimental design make it difficult to be confident that the left prefrontal activation is, in fact, caused by inhibition. For example, inhibition should have occurred primarily on “no” trials that involved recent negative-probes, but the PET activations for the recent negatives condition reflect the processing of all trial types, including nonrecent negative probes and “yes” responses. Also, inhibition should occur during only one temporal period of a trial with a recent negative probe—that period when the probe is presented—yet, the PET activations reflect all events that occur on a trial. In short, inhibition should have occurred primarily on specific trials at specific times, but the limited temporal resolution of PET (≈60 s) makes it impossible to test these claims.
We can, however, test these claims with fMRI as an imaging modality. Accordingly, we performed a follow-up experiment in which we used just the recent negatives condition§. We took advantage of recently developed techniques that allow fMRI images to be analyzed on a single-trial basis (41); this permits us to analyze images separately for trials that contain recent negative probes from those that contain nonrecent negative probes. To allow us to isolate different temporal periods within a trial, we used echoplanar imaging and increased the delay period from 3,000 to ≈7,000 ms and the intertrial interval from 3,00 to 14,500 ms; this made it possible to isolate activations for the target–presentation period, the delay period (when storage and rehearsal processes occur), or the probe period (when inhibition occurs).
The results of interest are the activations on negative-probe trials, particularly in a region of interest centered around the prefrontal activation observed in the previous PET study. Within that region, all seven subjects tested showed significantly more activation on recent than nonrecent negative probes. Further, the difference between the two types of negative probes obtained only during the probe period—roughly, from when the probe was presented until 5,000–6,000 ms later (a period long enough to take into account the slowness of the hemodynamic response). This result, too, was true of all subjects. These results strengthen our claims that inhibition is mediated by the prefrontal cortex and is dissociable from storage and rehearsal processes.
Some issues remain, though, in specifying the function of the prefrontal area of interest. One issue stems from a couple of additional findings in our fMRI experiment. The prefrontal area was activated during the probe interval even with nonrecent negative probes (just significantly less so than with recent negative probes). The prefrontal area also was activated during the interval when the target letters were presented (equally so for trials that would eventuate with recent or nonrecent negative probes) but not during the delay period. Thus, in addition to being activated when inhibition occurred, the prefrontal area also was activated whenever letters were presented (a result that was not observed in the prior PET study of inhibition because activations related to inputs were subtracted out). What is the function of these input-related activations? One possibility is that they again reflect an inhibitory metaprocess that damps a familiarity process or its outcome. That is, when a letter is presented, the familiarity level of its representation is increased; because familiarity level is an invalid guide to recognition decisions in our task, the familiarity process or its outcome may be inhibited, with more inhibition being generated when the familiarity process is in competition with another judgmental process. Alternatively, the input-related activations may have nothing to do with inhibition and instead may reflect some other aspect of input processing; in this case, there would be two functionally distinct regions in the prefrontal area of interest. Further research should be able to distinguish between these alternatives.
A final interpretive issue concerns the relation of our findings to previous ones. Some neuroimaging studies that have used competition–inhibition tasks have found frontal activations centered in the anterior cingulate, a structure that is substantially anterior and medial compared with the site of our prefrontal activation (36, 42, 43, 44). The reason for the different results with different inhibition tasks may lie in where in the processing sequence inhibition occurs. In our task, presumably, inhibition occurs at points where familiarity is considered, which is before any response is readied. In contrast, in some of the other tasks used in this research domain (e.g., the Stroop test, in which one must name the print color of a word and ignore the name of the word), inhibition may occur further downstream, at the level of programming or initiating a response. This issue, too, seems readily amenable to further research.
Acknowledgments
This work was supported by grants from the Department of Energy, McDonnel–Pew Program in Cognitive Neuroscience, National Institute of Aging, and Office of Naval Research.
ABBREVIATIONS
- WM
working memory
- PET
positron emission tomography
- fMRI
functional magnetic resonance imaging
Footnotes
Reuter-Lorenz, P. A., Miller, A. C., Gmeindl, L. & Rosier, E. Paper presented at Aging and Cognition Society Meetings, 1998, Atlanta, GA.
Rubenstein, J. S., Meyer, D. E. & Evans, J. Paper presented at Psychology Society Meetings, 1994, St. Louis, MO.
D’Esposito, M., Postle, B. R., Jonides, J., Smith. E. E., Leane, J. & Marshuetz, C. Poster presentation at Cognitive Neuroscience Society Meetings, 1998, San Francisco, CA.
References
- 1. Baddeley A D. Science. 1992;225:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter P A, Just M A, Shell P. Psychol Rev. 1990;97:404–431. [PubMed] [Google Scholar]
- 3.Jonides J. In: An Invitation to Cognitive Science: Thinking. Smith E E, Osherson D, editors. Vol. 3. Cambridge, MA: MIT Press; 1995. pp. 215–265. [Google Scholar]
- 4.Jonides J, Reuter-Lorenz P, Smith E E, Awh E, Barnes L, Drain M, Glass J, Lauber E, Patalano A, Schumacher E H. In: The Psychology of Learning and Motivation. Medin D, editor. New York: Academic; 1996. pp. 43–88. [Google Scholar]
- 5.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 6.Jonides J, Schumacher E H, Smith E E, Lauber E J, Awh E, Minoshima S, Koeppe R A. J Cognit Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 7.Baddeley A. Working Memory. Oxford: Oxford Univ. Press; 1986. [Google Scholar]
- 8.Awh E, Jonides J, Smith E E, Schumacher E H, Koeppe R A, Katz S. Psychol Sci. 1996;7:125–131. [Google Scholar]
- 9.Sternberg S. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 10.Fuster J M. Memory in the Cerebral Cortex. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 11.Cohen J D, Forman S D, Braver T S, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J D, Perlstein W M, Braver T S, Nystrom L E, Noll D C, Jonides J, Smith E E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 13.Smith E E. J Cognit Neurosci. 1997;9:167–169. doi: 10.1162/jocn.1997.9.1.167. [DOI] [PubMed] [Google Scholar]
- 14.Geschwind N. Scientific American. 1979;241:180–199. doi: 10.1038/scientificamerican0979-180. [DOI] [PubMed] [Google Scholar]
- 15.Cermak L S, Tarlow S. Cortex. 1978;14:32–40. doi: 10.1016/s0010-9452(78)80005-7. [DOI] [PubMed] [Google Scholar]
- 16.Shallice T. From Neuropsychology To Mental Structure. Cambridge. U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 17.Cohen S. Conduction Aphasia. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- 18.Anderson J. Architecture of Cognition. Cambridge, MA: Harvard Univ. Press; 1983. [Google Scholar]
- 19.Friedman R B. Cognit Neuropsychol. 1996;13:869–885. [Google Scholar]
- 20.Fiez J, Petersen S E. Proc Natl Acad Sci USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rypma, B., Prabhakaran, V., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. E. (1998) Neuroimage, in press. [DOI] [PubMed]
- 22.McElree B, Dosher B A. Exp Psychol General. 1989;118:346–373. [Google Scholar]
- 23.Monsell S. Cognit Psychol. 1978;10:465–501. [Google Scholar]
- 24.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintun M. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 25.Smith E E, Jonides J. Cognit Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 26.Kolb B, Whishaw I Q. Fundamentals of Human Neuropsychology. New York: Freeman; 1995. pp. 423–430. [Google Scholar]
- 27.Awh E, Jonides J. In: The Attentive Brain. Parasuraman R, editor. Cambridge, MA: MIT Press; 1996. pp. 353–380. [Google Scholar]
- 28.Courtney S M, Petit L, Maisog J, Ungerleider L G, Haxby J. Science. 1998;279:1347–1349. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 29.Awh E, Jonides J, Reuter-Lorenz P A. J Exp Psychol. 1998;24:780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- 30.Awh E, Jonides J, Smith E E, Hillyard S A, Anllo-Vento L R, Love T, Buxton R B, Wong E C, Swinney D. Soc Neurosci Abstr. 1997;23:296. [Google Scholar]
- 31.Heinze H J, Mangun G R, Burchert W, Hinrichs H, Scholz M, Munte T F, Gös A, Scherg M, Johannes S, Hundeshagen H, et al. Nature (London) 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J D, Servan-Schrieber D. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Meyer D E, Kieras D. Psychol Rev. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 34.Norman D A, Shallice T. In: Conscious and Self-Regulation: Advances in Research and Theory. Schwartz G E, Shapiro D, editors. New York: Plenum; 1986. [Google Scholar]
- 35.Diamond A, Cruttenden L, Neiderman D. Dev Psychol. 1994;30:192–205. [Google Scholar]
- 36.Pardo J V, Pardo J, Janer W, Raichle M E. Proc Natl Acad Sci USA. 1990;95:876–882. [Google Scholar]
- 37.D’Esposito M, Detre J A, Alsop D C, Shin R K, Atlas S, Grossman M. Nature (London) 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 38.Smith E E, Jonides J, Marshuetz C, Koeppe R A. Proc Natl Acad Sci USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonides J, Smith E E, Marshuetz C, Koeppe R A, Reuter-Lorenz P A. Proc Natl Acad Sci USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuter-Lorenz P, Jonides J, Smith E E, Hartley A A, Cianciolo A, Awh E, Marshuetz C, Koeppe R A. Soc Neurosci Abstr. 1996;22:183. [Google Scholar]
- 41.Zarahn E, Aguirre G K, D’Esposito M. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 42.Frith C D, Friston K J, Liddle P F, Frackowiack R S J. Neuropsychologica. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- 43.Taylor S F, Kornblum S, Lauber E J, Minoshima S, Koeppe R A. Neuroimage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- 44.Carter C S, Braver T S, Barch D M, Botvinick M M, Noll D, Cohen J D. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]