Abstract
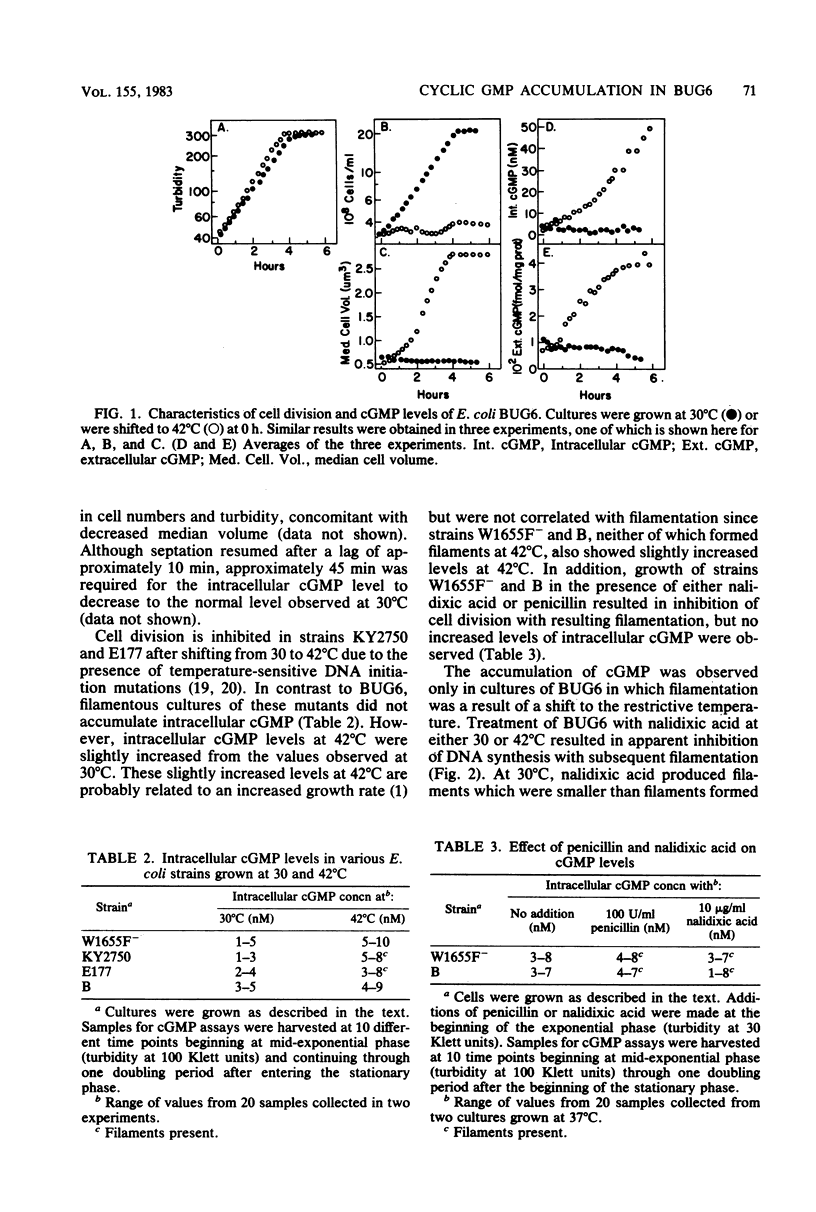
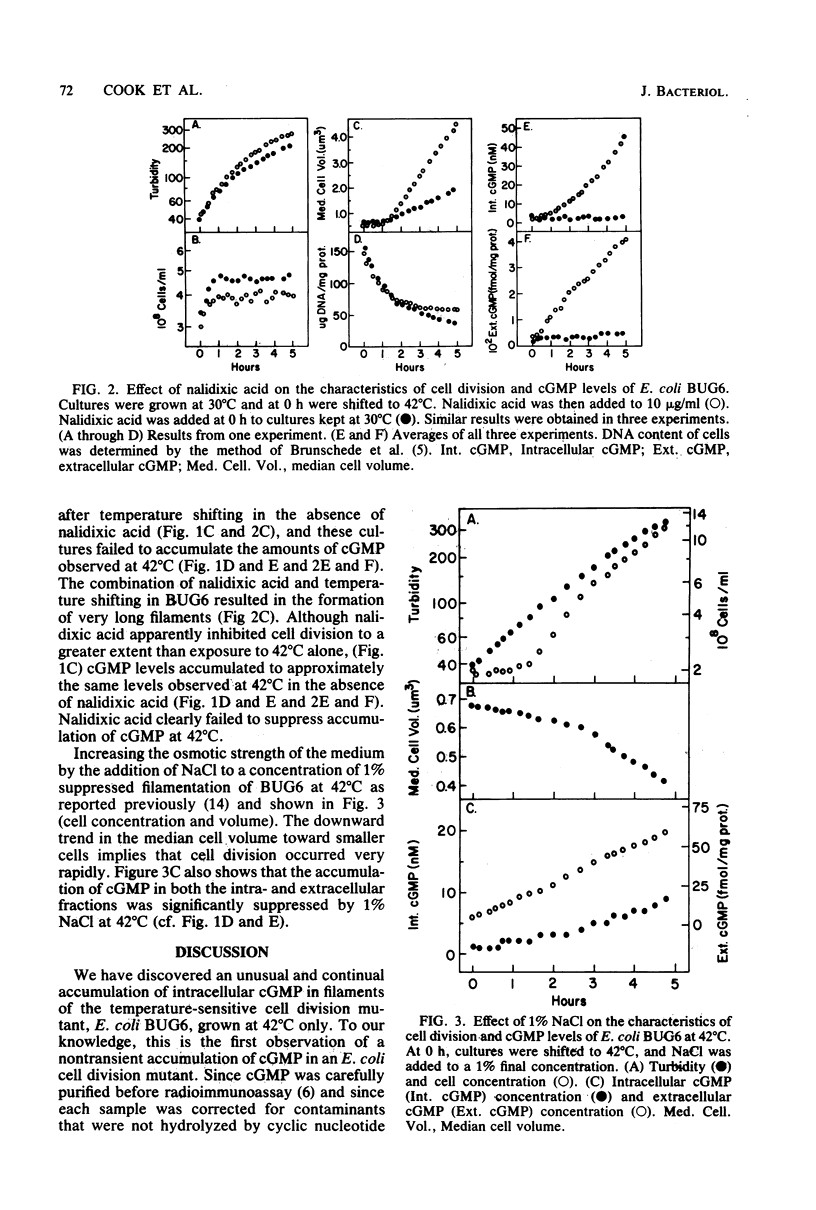
Experiments with Escherichia coli BUG6, a temperature-sensitive cell division mutant, have shown that at the restrictive temperature (42 degrees C) the loss of cell division potential (filamentation) was accompanied by an unusual increase in intracellular cyclic GMP (cGMP). At the permissive temperature (30 degrees C), cell division proceeded normally, and cGMP did not accumulate. Increasing the osmotic strength of the medium with NaCl suppressed filamentation in BUG6 at 42 degrees C and also suppressed the temperature-sensitive accumulation of cGMP. The addition of nalidixic acid to BUG6 at 30 degrees C induced filamentation but failed to cause cGMP accumulation. A similar accumulation of cGMP has not been observed in other E. coli strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Involvement of cyclic GMP in intracellular signaling in the chemotactic response of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3879–3883. doi: 10.1073/pnas.77.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Brunschede H., Dove T. L., Bremer H. Establishment of exponential growth after a nutritional shift-up in Escherichia coli B/r: accumulation of deoxyribonucleic acid, ribonucleic acid, and protein. J Bacteriol. 1977 Feb;129(2):1020–1033. doi: 10.1128/jb.129.2.1020-1033.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. R., Kalb V. F., Jr, Peace A. A., Bernlohr R. W. Is cyclic guanosine 3',5'-monophosphate a cell cycle regulator? J Bacteriol. 1980 Mar;141(3):1450–1453. doi: 10.1128/jb.141.3.1450-1453.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. Accumulation of cyclic guanosine 3':5'-monophosphate in the culture medium of growing cells of Escherichia coli. Biochem Biophys Res Commun. 1976 Jan 26;68(2):430–435. doi: 10.1016/0006-291x(76)91163-3. [DOI] [PubMed] [Google Scholar]
- Lederberg E M, Lederberg J. Genetic Studies of Lysogenicity in Escherichia Coli. Genetics. 1953 Jan;38(1):51–64. doi: 10.1093/genetics/38.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Hennecke H., Scott D. B. Effect of cyclic guanosine 3',5'-monophosphate on nitrogen fixation in Rhizobium japonicum. J Bacteriol. 1979 Jul;139(1):256–263. doi: 10.1128/jb.139.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia V., Caputo G., Mandato E., Rocino A., Adhya S., Pastan I. Guanylate cyclase activity in Escherichia coli mutants defective in adenylate cyclase. J Bacteriol. 1981 Sep;147(3):931–934. doi: 10.1128/jb.147.3.931-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun I. Y., Shapiro L., Rosen O. M. A specific cyclic guanosine 3':5'-monophosphate-binding protein in Caulobacter crescentus. J Biol Chem. 1975 Aug 10;250(15):6181–6184. [PubMed] [Google Scholar]
- Utsumi R., Tanabe H., Nakamoto Y., Kawamukai M., Sakai H., Himeno M., Komano T., Hirota Y. Inhibitory effect of adenosine 3',5'-phosphate on cell division of Escherichia coli K-12 mutant derivatives. J Bacteriol. 1981 Sep;147(3):1105–1109. doi: 10.1128/jb.147.3.1105-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]



