Abstract
Bacillopeptidase F is a serine endopeptidase excreted by Bacillus subtilis 168 after the end of exponential growth. As a step toward discovering a physiological function for this protease, an enzymological and immunological study was undertaken. When bacillopeptidase F was purified at pH 10, a number of enzymically active, rapidly moving electrophoretic forms were observed, as had been previously reported. Rabbit antiserum was prepared against one form. When the enzyme was purified at pH 6.0 in the presence of the covalent inhibitor phenylmethylsulfonyl fluoride, using the rabbit antiserum to detect the bacillopeptidase F protein, no fast-moving electrophoretic forms were observed. Instead, only two forms of the enzyme were isolated. One form had a molecular weight of 33,000, and the other had a molecular weight of 50,000, as determined by equilibrium sedimentation methods. Both forms appeared to be glycoproteins, both contained compounds, released on acid hydrolysis, which cochromatographed with phosphoserine and galactosamine, and the two gave identical immunoprecipitin lines in Ouchterlony double-diffusion tests. The smaller form had a pI of 4.4, whereas the larger had a pI of 5.4. The data suggest that bacillopeptidase F is distinct from all other proteases of B. subtilis.
Full text
PDF


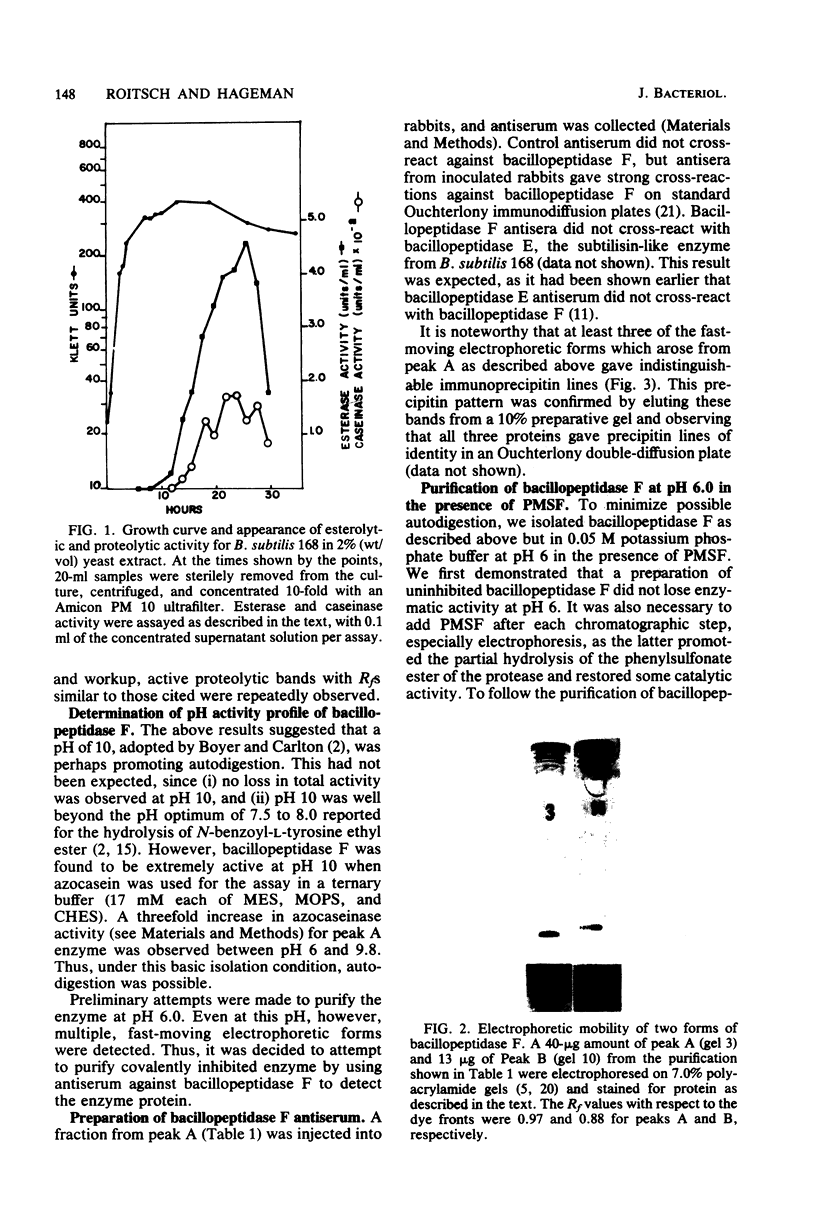

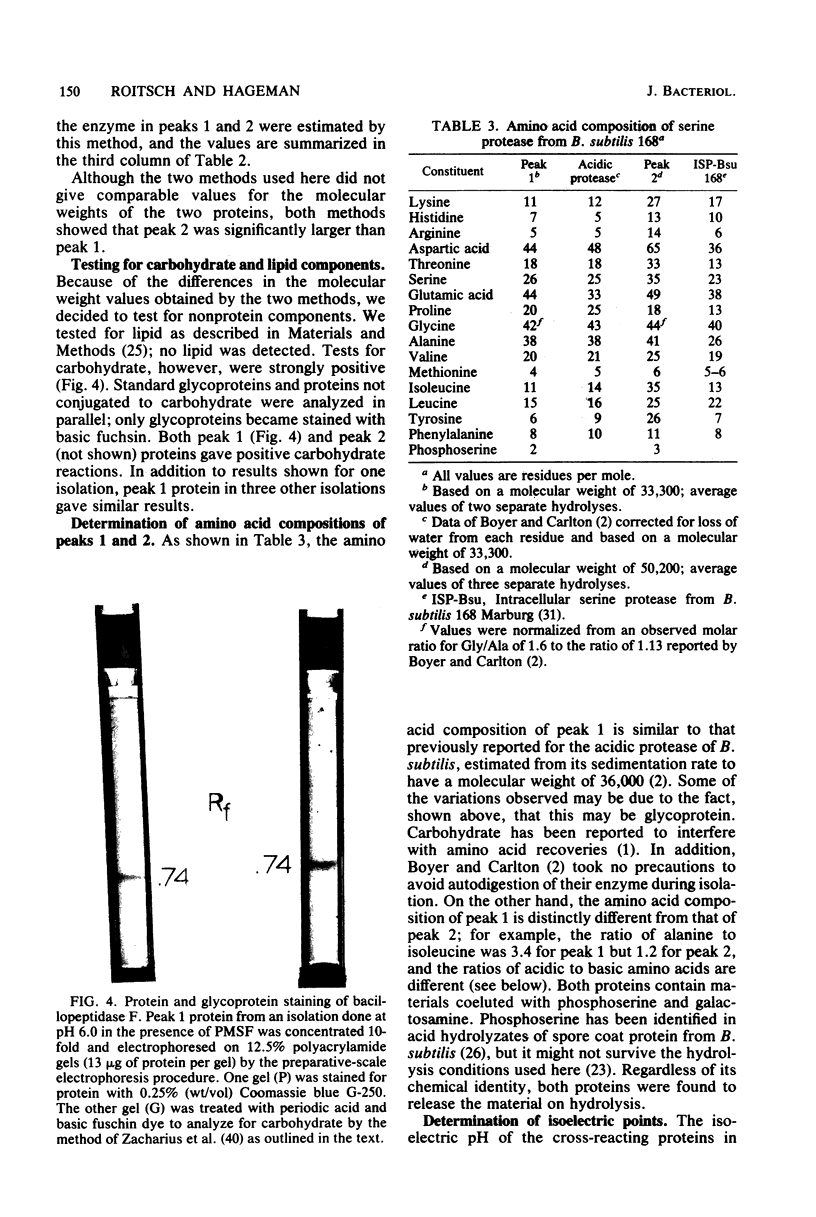


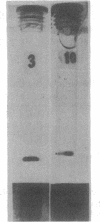
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Frehel C., Dubray G., Wolff A. Activité estérasique des membranes cytoplasmiques et mésosomiques au cours de la sporulation de Bacillus subtilis. Biochimie. 1974;56(4):583–598. doi: 10.1016/s0300-9084(74)80077-5. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. An enzymatic and immunological comparison of two proteases from a transformable Bacillus subtilis with the "subtilisins". Arch Biochem Biophys. 1970 Jul;139(1):67–79. doi: 10.1016/0003-9861(70)90045-7. [DOI] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. Effects of mutational loss of specific intracellular proteases on the sporulation of Bacillus subtilis. J Bacteriol. 1973 May;114(2):612–617. doi: 10.1128/jb.114.2.612-617.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mamas S., Millet J. Purification et propriétés d'une estérase excrétée pendant la sporulation de Bacillus subtilis. Biochimie. 1975;57(1):9–16. doi: 10.1016/s0300-9084(75)80104-0. [DOI] [PubMed] [Google Scholar]
- Millet J. Caractérisation de deux endopeptidases excrétées par B. subtilis Marburg au cours de la sporulation. Bull Soc Chim Biol (Paris) 1969 Jul 25;51(3):457–469. [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Extracellular and membrane-bound proteases from Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):493–501. doi: 10.1128/jb.141.2.493-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimmer R. H. Esters of phosphoric acid: Phosphoryl hydroxyamino-acids. Biochem J. 1941 Apr;35(4):461–469. doi: 10.1042/bj0350461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Ichikawa T., Kondo M. Occurrence of phosphorylserine in the spore coat of Bacillus subtilis NRRL B558. Microbios. 1975;12(47-48):67–76. [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava O. P., Aronson A. I. Isolation and characterization of a unique protease from sporulating cells of Bacillus subtilis. Arch Microbiol. 1981 May;129(3):227–232. doi: 10.1007/BF00425256. [DOI] [PubMed] [Google Scholar]
- Stepanov V. M., Strongin A. Y., Izotova L. S., Abramov Z. T., Lyublinskaya L. A., Ermakova L. M., Baratova L. A., Belyanova L. P. Intracellular serine protease from Bacillus subtilis. Structural comparison with extracellular serine proteases-subtilisins. Biochem Biophys Res Commun. 1977 Jul 11;77(1):298–305. doi: 10.1016/s0006-291x(77)80196-4. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Gorodetsky D. I., Kuznetsova I. A., Yanonis V. V., Abramov Z. T., Belyanova L. P., Baratova L. A., Stepanov V. M. Intracellular serine proteinase of Bacillus subtilis strain Marburg 168. Comparison with the homologous enzyme from Bacillus subtilis strain A-50. Biochem J. 1979 May 1;179(2):333–339. doi: 10.1042/bj1790333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Ermakova L. M., Gorodetsky D. I., Stepanov V. M. On the appearance of Bacillus subtilis intracellular serine protease in the cell membrane and culture medium. Comparison of the enzyme and other Bacillus subtilis serine proteases. Arch Microbiol. 1978 Dec 20;119(3):287–293. doi: 10.1007/BF00405408. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Uemitsu N., Sugiyama M., Matsumiya H. The reactivation of phenylmethanesulfonyl-subtilisin. Biochim Biophys Acta. 1972 Feb 28;258(2):562–565. doi: 10.1016/0005-2744(72)90248-3. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Gaucher G. M., Roche R. S. Anomalous molecular weights to proteases in gel chromatography. Biochem Biophys Res Commun. 1974 May 7;58(1):8–12. doi: 10.1016/0006-291x(74)90883-3. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]