Abstract
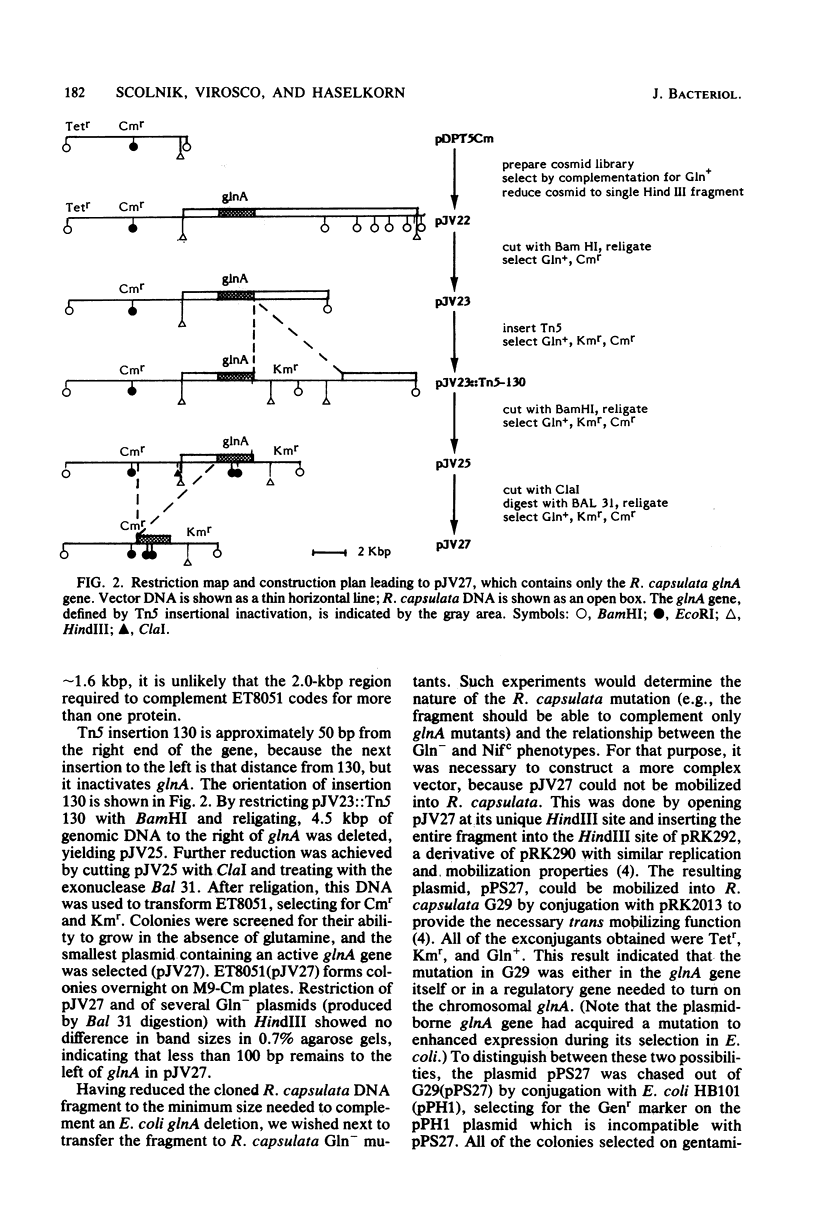
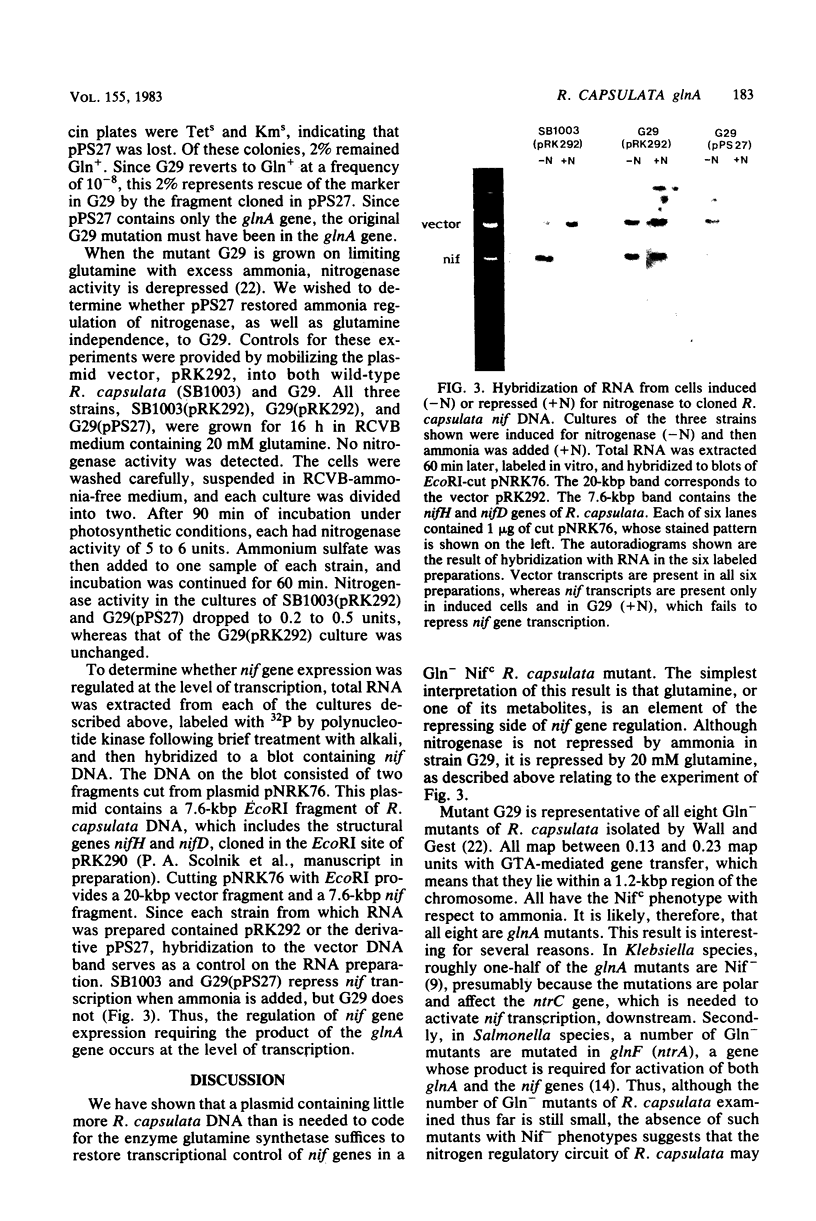
The wild-type glnA gene, coding for glutamine synthetase, was cloned from the photosynthetic bacterium Rhodopseudomonas capsulata by using a cosmid library to complement the Gln- phenotype of an Escherichia coli glnA deletion strain. The original cosmid plasmid contained 37 kilobase pairs (kbp) of R. capsulata DNA, of which only 2 kbp was necessary for Gln complementation in E. coli. A plasmid containing this 2-kbp insert was mobilized into G29, a Gln- mutant of R. capsulata which is also unable to repress nitrogenase in ammonia-containing media (Nifc phenotype). The 2-kbp fragment restored glutamine-independent growth and ammonia repression of nitrogenase, indicating that in R. capsulata, production of the signal for nitrogen repression of nif depends on the activity of the glnA gene. Repression of nitrogenase was shown, by hybridization of RNA to cloned nif DNA, to occur at the level of transcription in the wild-type and the complemented G29 strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins J. Escherichia coli plasmids packageable in vitro in lambda bacteriophage particles. Methods Enzymol. 1979;68:309–326. doi: 10.1016/0076-6879(79)68022-9. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol. 1982 Feb;149(2):708–717. doi: 10.1128/jb.149.2.708-717.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Adenylylation/deadenylylation control of the glutamine synthetase of Rhodopseudomonas capsulata. Eur J Biochem. 1977 Dec 1;81(2):365–371. doi: 10.1111/j.1432-1033.1977.tb11960.x. [DOI] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Inorganic nitrogen assimilation by the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1976 Nov;128(2):683–688. doi: 10.1128/jb.128.2.683-688.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo J. M., Goldberg R. B. Regulation of nitrogen metabolism in glutamine auxotrophs of Klebsiella pneumoniae. J Bacteriol. 1980 Apr;142(1):99–110. doi: 10.1128/jb.142.1.99-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Removal of an adenine-like molecule during activation of dinitrogenase reductase from Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6201–6205. doi: 10.1073/pnas.76.12.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Wall J. D., Gest H. Dark anaerobic dinitrogen fixation by a photosynthetic microorganism. Science. 1979 Jun 29;204(4400):1429–1430. doi: 10.1126/science.204.4400.1429. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merrick M. J. A new model for nitrogen control. Nature. 1982 Jun 3;297(5865):362–363. doi: 10.1038/297362a0. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta. 1977 Oct 12;462(1):187–195. doi: 10.1016/0005-2728(77)90201-8. [DOI] [PubMed] [Google Scholar]
- Pahel G., Bloom F. R., Tyler B. Deletion mapping of the polA-metB region of the Escherichia coli chromosome. J Bacteriol. 1979 May;138(2):653–656. doi: 10.1128/jb.138.2.653-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tuli R., Fisher R., Haselkorn R. The ntr genes of Escherichia coli activate the hut and nif operons of Klebsiella pneumoniae. Gene. 1982 Jul-Aug;19(1):109–116. doi: 10.1016/0378-1119(82)90195-0. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]