Abstract
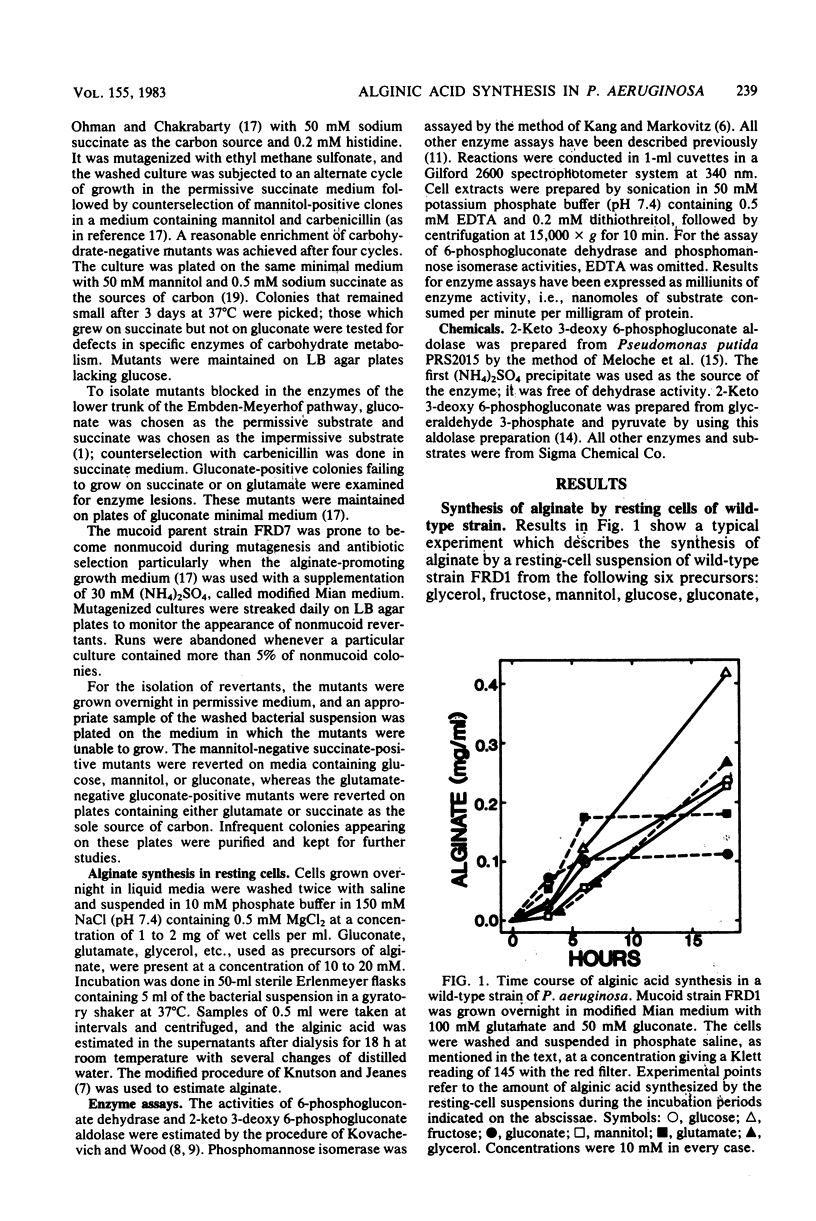
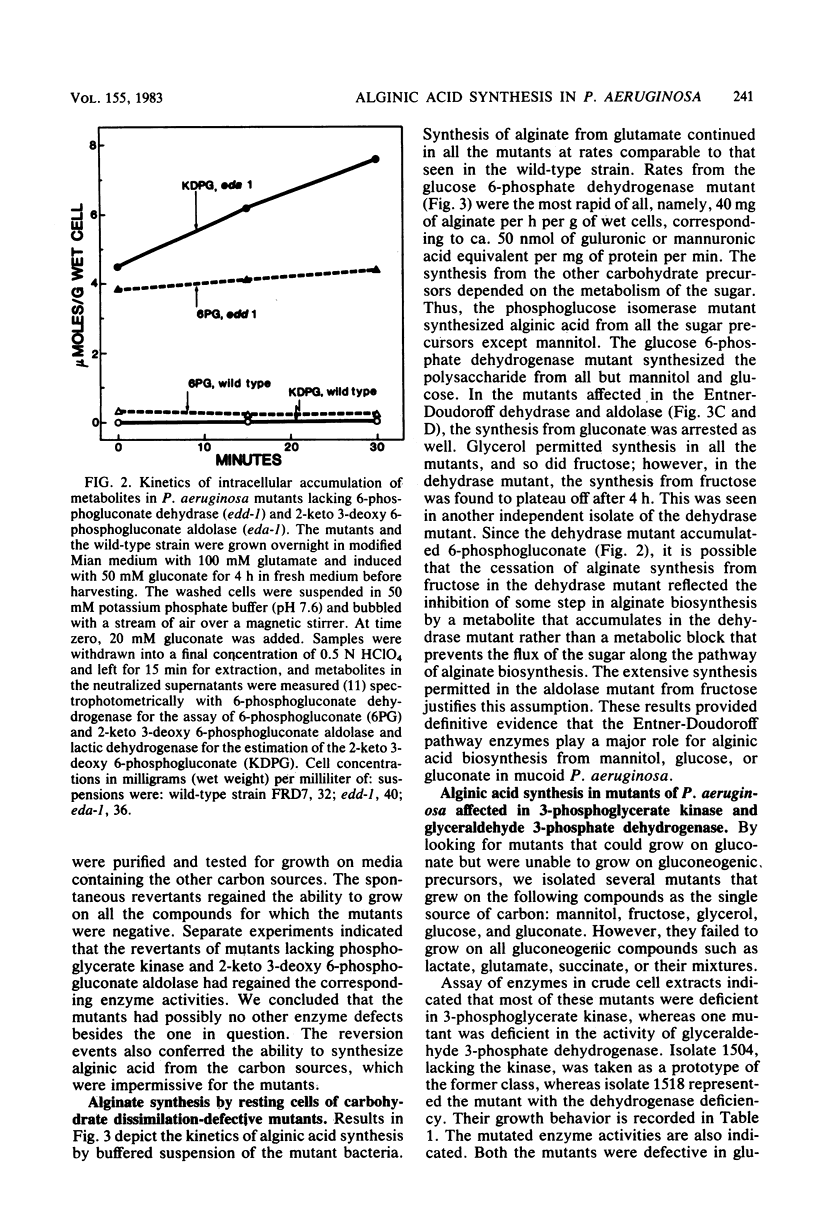
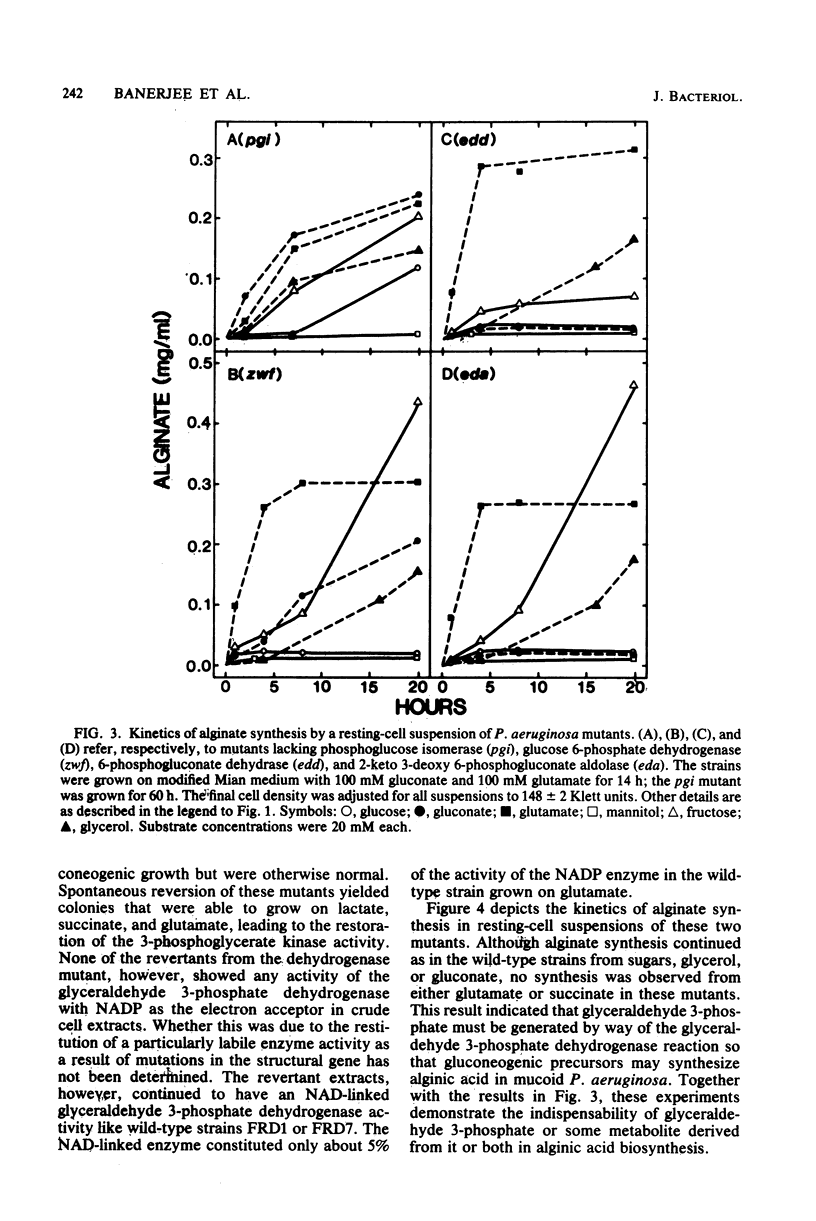
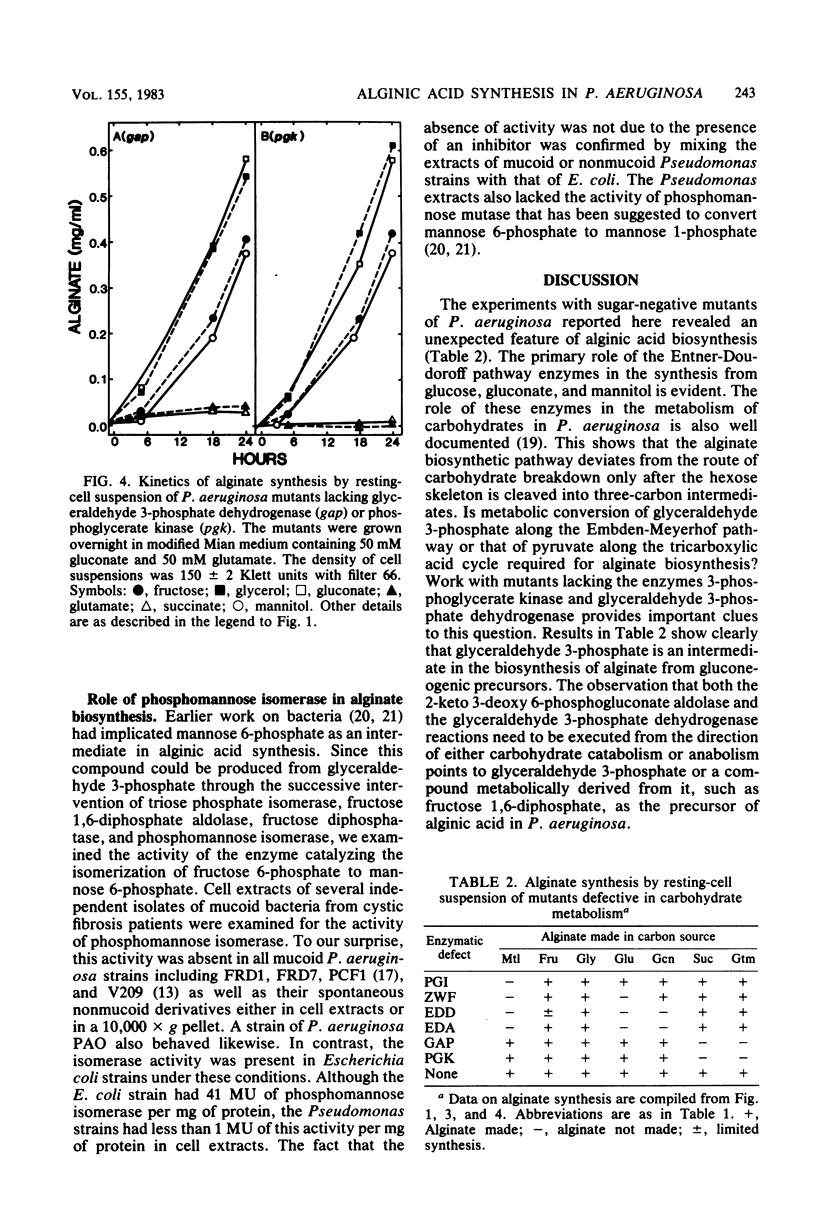
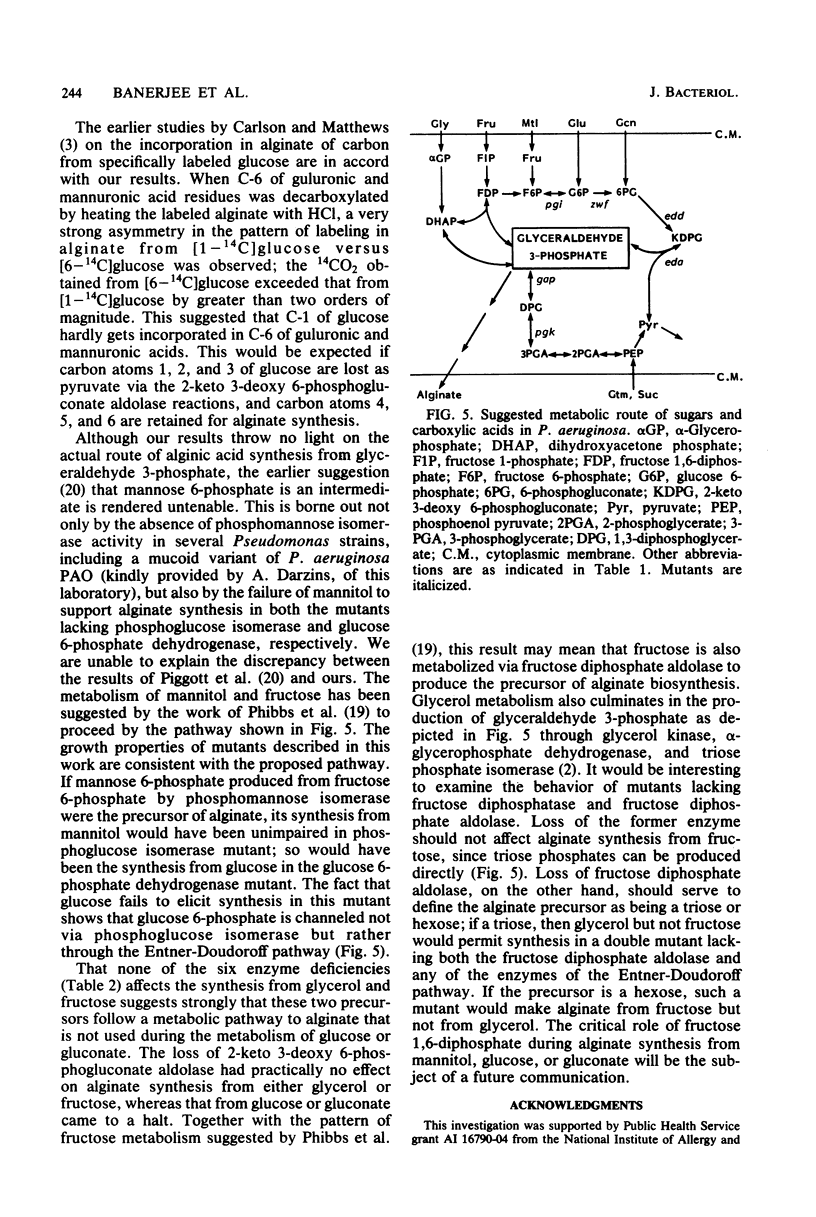
Mutant cells of mucoid Pseudomonas aeruginosa isolated from cystic fibrosis patients were examined for their ability to synthesize alginic acid in resting cell suspensions. Unlike the wild-type strain which synthesizes alginic acid from glycerol, fructose, mannitol, glucose, gluconate, glutamate, or succinate, mutants lacking specific enzymes of carbohydrate metabolism are uniquely impaired. A phosphoglucose isomerase mutant did not synthesize the polysaccharide from mannitol, nor did a glucose 6-phosphate dehydrogenase mutant synthesize the polysaccharide from mannitol or glucose. Mutants lacking the Entner-Doudoroff pathway dehydrase or aldolase failed to produce alginate from mannitol, glucose, or gluconate, as a 3-phosphoglycerate kinase or glyceraldehyde 3-phosphate dehydrogenase mutant failed to produce from glutamate or succinate. These results demonstrate the primary role of the Entner-Doudoroff pathway enzymes in the synthesis of alginate from glucose, mannitol, or gluconate and the role of glyceraldehyde 3-phosphate dehydrogenase reaction for the synthesis from gluconeogenic precursors such as glutamate. The virtual absence of any activity of phosphomannose isomerase in cell extracts of several independent mucoid bacteria and the impairment of alginate synthesis from mannitol in mutants lacking phosphoglucose isomerase or glucose 6-phosphate dehydrogenase rule out free mannose 6-phosphate as an intermediate in alginate biosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio M. L., Ruiz-Amil M., Vicente M., Cánovas J. L. The role of phosphoglycerate kinase in the metabolism of Pseudomonas putida. FEBS Lett. 1971 May 20;14(5):326–328. doi: 10.1016/0014-5793(71)80292-2. [DOI] [PubMed] [Google Scholar]
- Blevins W. T., Feary T. W., Phibbs P. V., Jr 6-Phosphogluconate dehydratase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1975 Mar;121(3):942–949. doi: 10.1128/jb.121.3.942-949.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M., Matthews L. W. Polyuronic acids produced by Pseudomonas aeruginosa. Biochemistry. 1966 Sep;5(9):2817–2822. doi: 10.1021/bi00873a006. [DOI] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. III. Purification and properties of a 6-phosphogluconate dehydrase. J Biol Chem. 1955 Apr;213(2):745–756. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. IV. Purification and properties of 2-keto-3-deoxy-6-phosphogluconate aldolase. J Biol Chem. 1955 Apr;213(2):757–767. [PubMed] [Google Scholar]
- Kang S., Markovitz A. Induction of capsular polysaccharide synthesis by rho-fluorophenylalanine in Escherichia coli wild type and strains with altered phenylalanyl soluble ribonucleic acid synthetase. J Bacteriol. 1967 Feb;93(2):584–591. doi: 10.1128/jb.93.2.584-591.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Lin T. Y., Hassid W. Z. Pathway of algnic acid synthesis in the marine brown alga, Fucus gardneri Silva. J Biol Chem. 1966 Nov 25;241(22):5284–5297. [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. A kinetic study of glycolytic enzyme synthesis in yeast. J Biol Chem. 1971 Jan 25;246(2):475–488. [PubMed] [Google Scholar]
- Markowitz S. M., Macrina F. L., Phibbs P. V., Jr R-factor inheritance and plasmid content in mucoid Pseudomonas aeruginosa. Infect Immun. 1978 Nov;22(2):530–539. doi: 10.1128/iai.22.2.530-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Chakrabarty A. M. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun. 1981 Jul;33(1):142–148. doi: 10.1128/iai.33.1.142-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Chakrabarty A. M. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect Immun. 1982 Aug;37(2):662–669. doi: 10.1128/iai.37.2.662-669.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, McCowen S. M., Feary T. W., Blevins W. T. Mannitol and fructose catabolic pathways of Pseudomonas aeruginosa carbohydrate-negative mutants and pleiotropic effects of certain enzyme deficiencies. J Bacteriol. 1978 Feb;133(2):717–728. doi: 10.1128/jb.133.2.717-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindar D. F., Bucke C. The biosynthesis of alginic acid by Azotobacter vinelandii. Biochem J. 1975 Dec;152(3):617–622. doi: 10.1042/bj1520617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis of microbial exopolysaccharides. Adv Microb Physiol. 1982;23:79–150. doi: 10.1016/s0065-2911(08)60336-7. [DOI] [PubMed] [Google Scholar]



