Abstract
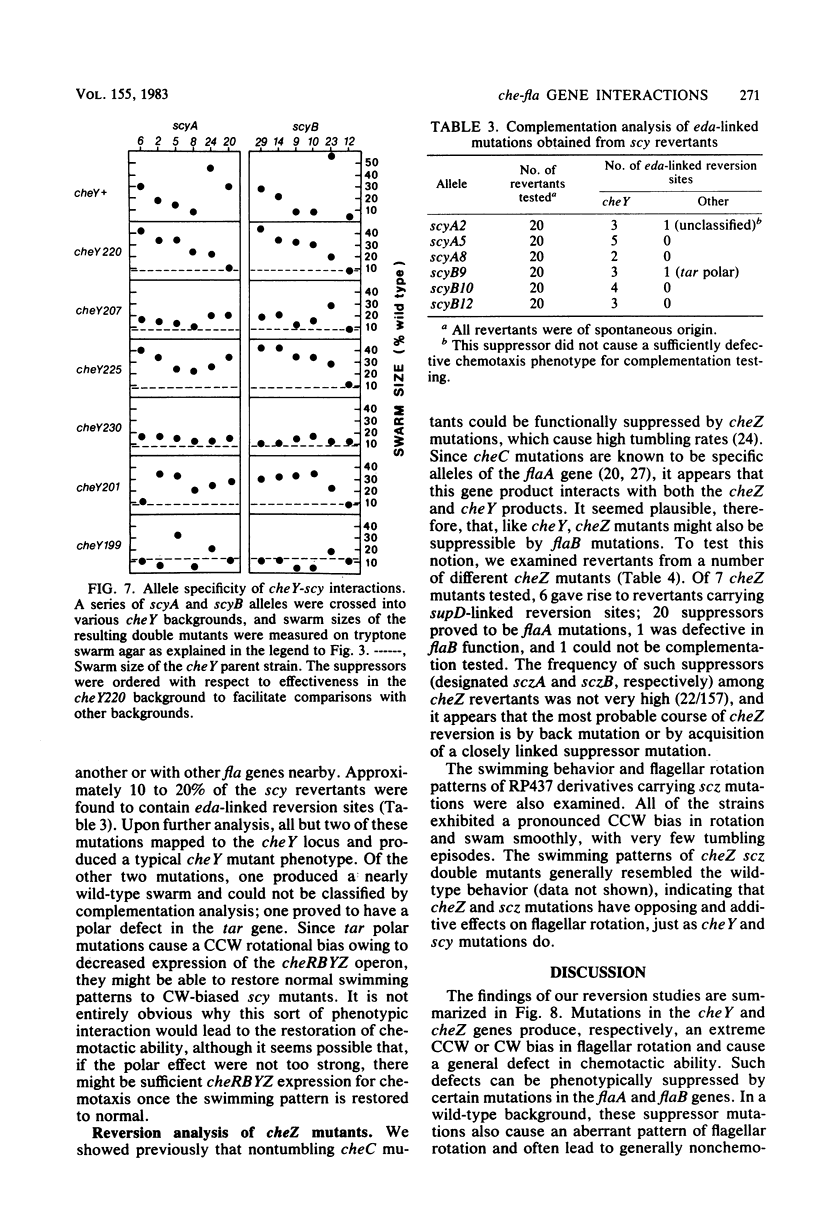
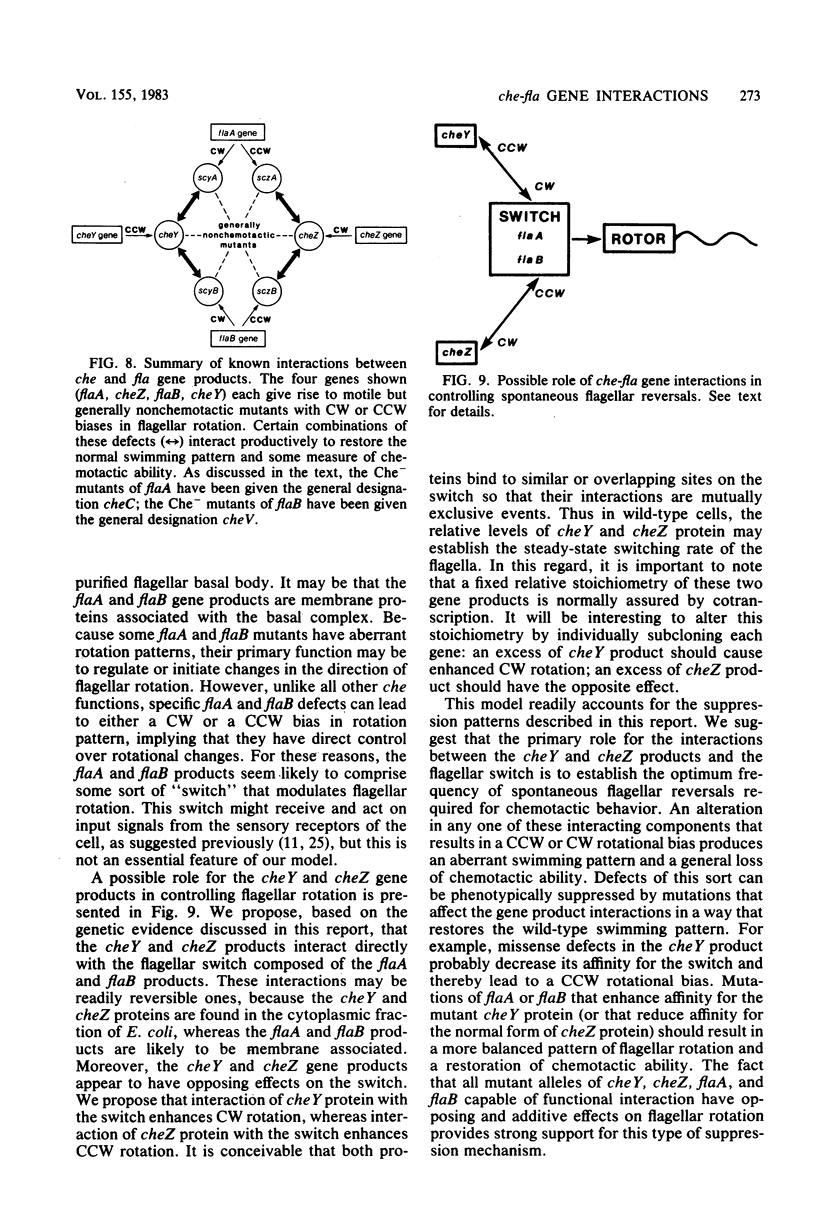
Escherichia coli mutants defective in cheY and cheZ function are motile but generally nonchemotactic; cheY mutants have an extreme counterclockwise bias in flagellar rotation, whereas cheZ mutants have a clockwise rotational bias. Chemotactic pseudorevertants of cheY and cheZ mutants were isolated on semisolid agar and examined for second-site suppressors in other chemotaxis-related loci. Approximately 15% of the cheZ revertants and over 95% of the cheY revertants contained compensatory mutations in the flaA or flaB locus. When transferred to an otherwise wild-type background, most of these suppressor mutations resulted in a generally nonchemotactic phenotype: suppressors of cheY caused a clockwise rotational bias; suppressors of cheZ produced a counterclockwise rotational bias. Chemotactic double mutants containing a che and a fla mutation invariably exhibited flagellar rotation patterns in between the opposing extremes characteristic of the component mutations. This additive effect on flagellar rotation resulted in essentially wild-type swimming behavior and is probably the major basis of suppressor action. However, suppression effects were also allele specific, suggesting that the cheY and cheZ gene products interact directly with the flaA and flaB products. These interactions may be instrumental in establishing the unstimulated swimming pattern of E. coli.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. B., Adler J. Complementation of nonchemotactic mutants of Escherichia coli. Genetics. 1969 Jan;61(1):61–66. doi: 10.1093/genetics/61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Dynamic properties of bacterial flagellar motors. Nature. 1974 May 3;249(452):77–79. doi: 10.1038/249077a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Tedesco P. M. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. L., Stocker B. A. Salmonella typhimurium mutants generally defective in chemotaxis. J Bacteriol. 1976 Dec;128(3):754–765. doi: 10.1128/jb.128.3.754-765.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Construction and behavior of strains with mutations in two chemotaxis genes. J Bacteriol. 1982 Jun;150(3):1297–1301. doi: 10.1128/jb.150.3.1297-1301.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Molecular cloning of chemotaxis genes and overproduction of gene products in the bacterial sensing system. J Bacteriol. 1981 Aug;147(2):390–400. doi: 10.1128/jb.147.2.390-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Parkinson J. S., Koshland D. E., Jr Functional homology of chemotaxis genes in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1979 Jul;139(1):107–114. doi: 10.1128/jb.139.1.107-114.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G. E., Aizawa S. I., Macnab R. M. flaAII (motC, cheV) of Salmonella typhimurium is a structural gene involved in energization and switching of the flagellar motor. J Bacteriol. 1983 Apr;154(1):84–91. doi: 10.1128/jb.154.1.84-91.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Macnab R. M., DeFranco A. L., Koshland D. E., Jr Inversion of a behavioral response in bacterial chemotaxis: explanation at the molecular level. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4150–4154. doi: 10.1073/pnas.75.9.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Kutsukake K., Iino T. Definition of additional flagellar genes in Escherichia coli K12. Genetics. 1980 Feb;94(2):277–290. doi: 10.1093/genetics/94.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Shimada K., Iino T. Isolation of specialized lambda transducing bacteriophages for flagellar genes (fla) of Escherichia coli K-12. J Virol. 1977 Jun;22(3):654–661. doi: 10.1128/jvi.22.3.654-661.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H., Ozeki H. Deletion and amber mutants of fla loci in Escherichia coli K-12. Genetics. 1976 Nov;84(3):403–421. doi: 10.1093/genetics/84.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H. Tumbling chemotaxis mutants of Escherichia coli: possible gene-dependent effect of methionine starvation. J Bacteriol. 1980 May;142(2):527–534. doi: 10.1128/jb.142.2.527-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K., Iino T., Komeda Y., Yamaguchi S. Functional homology of fla genes between Salmonella typhimurium and Escherichia coli. Mol Gen Genet. 1980 Apr;178(1):59–67. doi: 10.1007/BF00267213. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978 Jul;135(1):45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Houts S. E. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982 Jul;151(1):106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R. Interaction of the cheC and cheZ gene products is required for chemotactic behavior in Escherichia coli. Proc Natl Acad Sci U S A. 1979 May;76(5):2390–2394. doi: 10.1073/pnas.76.5.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J Bacteriol. 1976 May;126(2):758–770. doi: 10.1128/jb.126.2.758-770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubik B. A., Koshland D. E., Jr Potentiation, desensitization, and inversion of response in bacterial sensing of chemical stimuli. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2820–2824. doi: 10.1073/pnas.75.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of bacteriophage Mu-induced flagellar mutants in Escherichia coli. J Bacteriol. 1973 Oct;116(1):114–122. doi: 10.1128/jb.116.1.114-122.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of flagellar mutants in Escherichia coli. J Bacteriol. 1973 Jan;113(1):105–113. doi: 10.1128/jb.113.1.105-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick H. M., Taylor B. L., Koshland D. E., Jr Chemotactic mechanism of Salmonella typhimurium: preliminary mapping and characterization of mutants. J Bacteriol. 1977 Apr;130(1):223–231. doi: 10.1128/jb.130.1.223-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




