Abstract
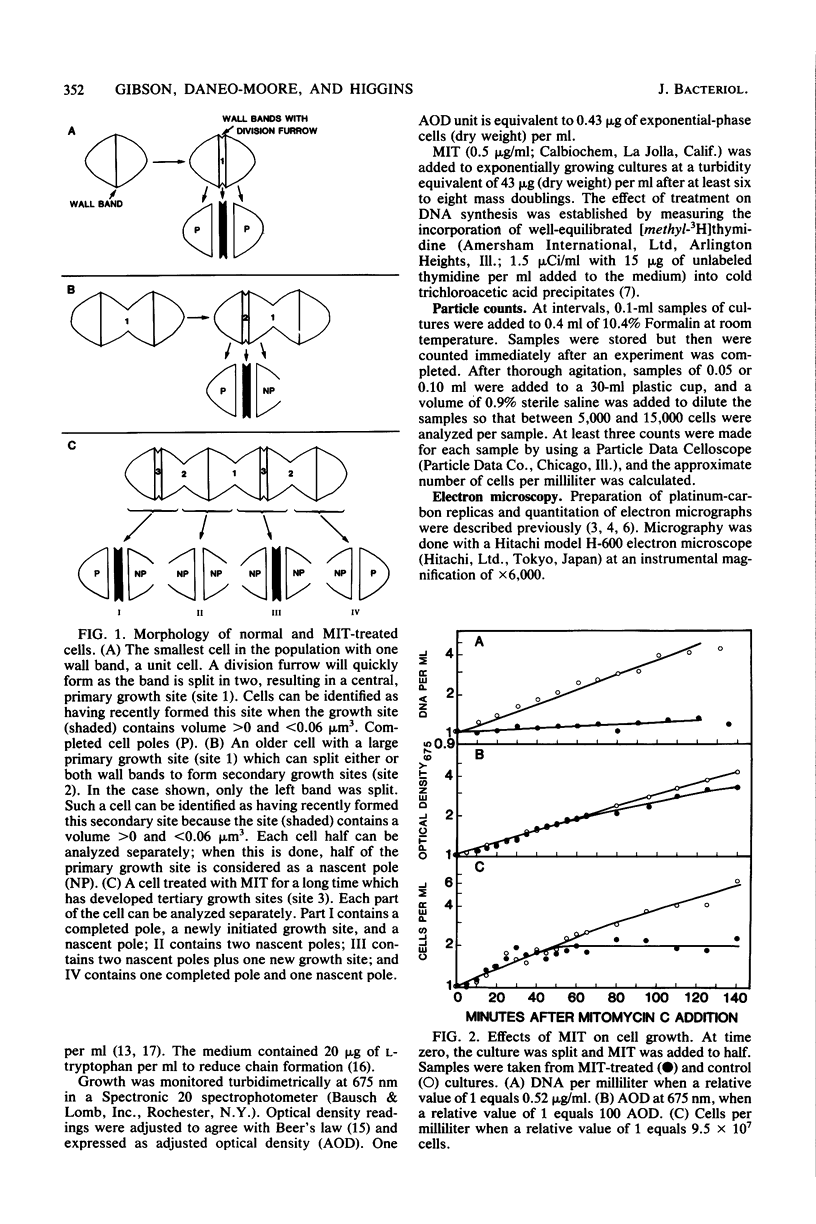
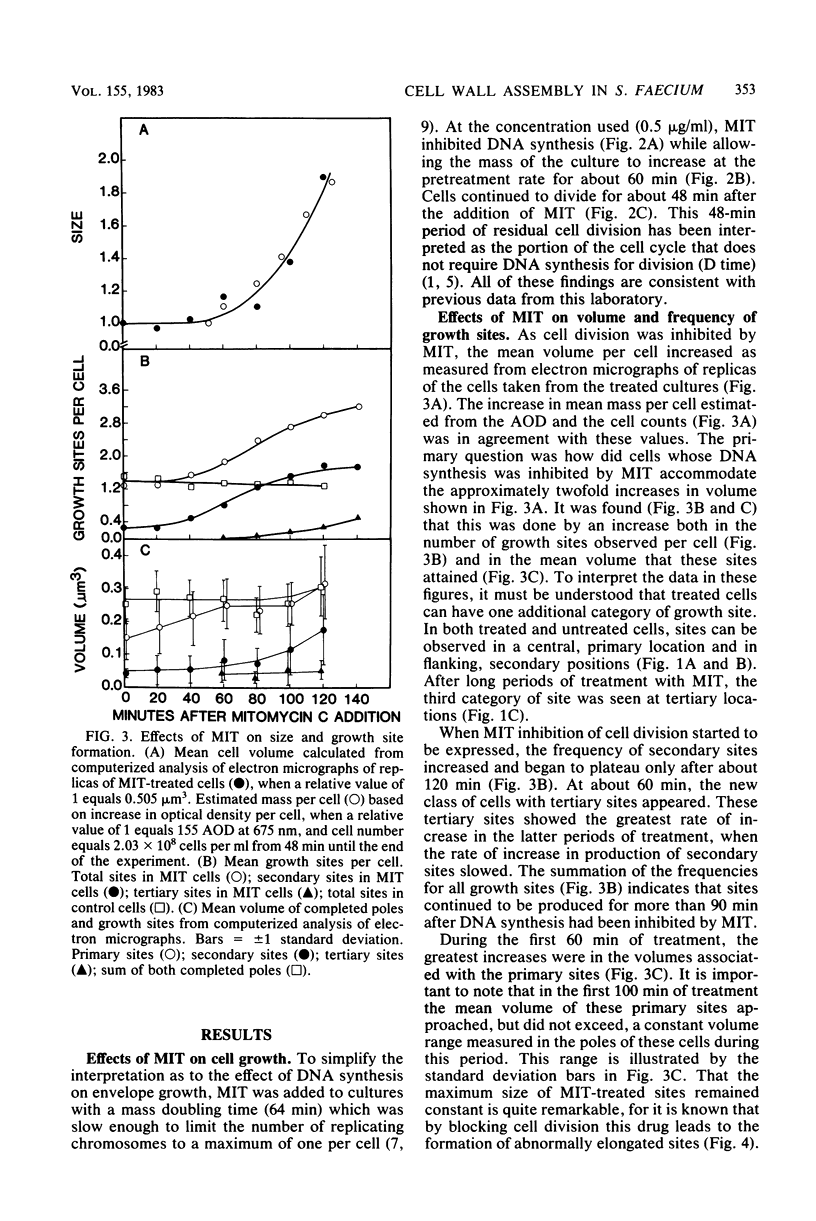
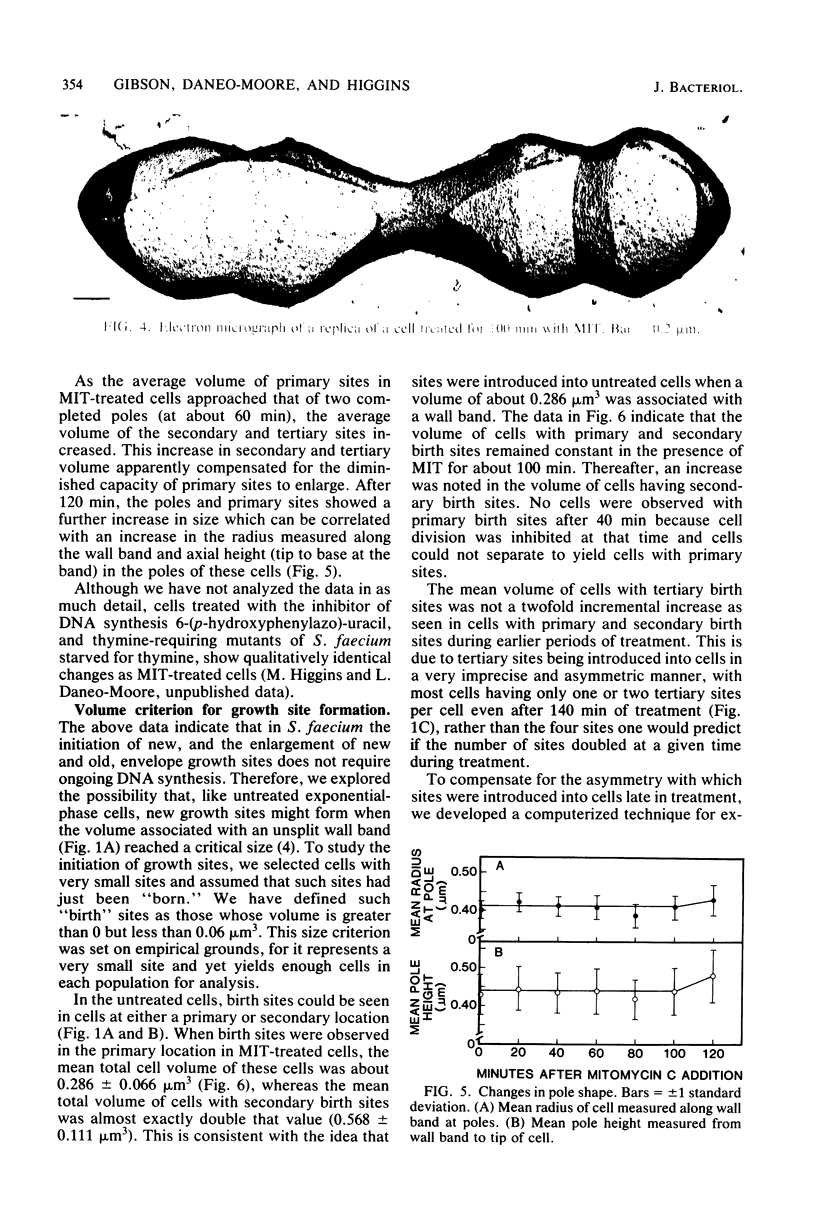
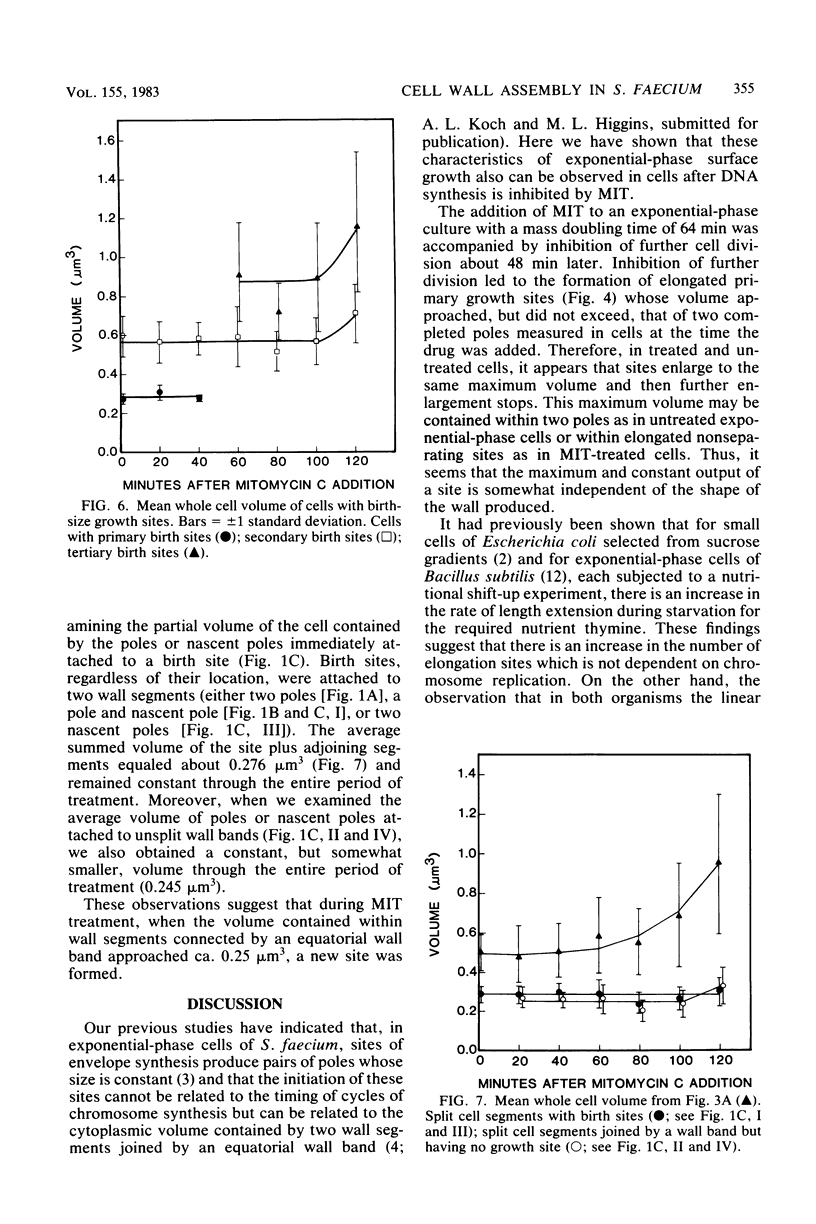
Growth sites which are bounded by raised wall bands can be observed in electron micrographs of replicas of Streptococcus faecium. When mitomycin C was added to an exponential-phase culture doubling in mass every 64 min, DNA synthesis was inhibited, and eventually cell division stopped. The growth sites formed before and after inhibition of DNA synthesis enlarged until they contained about 0.25 micron3 of cell volume, at which point they ceased to increase in size. When these sites approached this 0.25-micron3 limit, new sites were initiated; this result had also been observed in untreated cells undergoing a large range of exponential-phase mass doubling times. Thus, regardless of whether chromosome replication is inhibited or uninhibited, sites have the same finite capacity to enlarge to about 0.25 micron3, and when this capacity is reached, new sites are initiated. Although initiation of new growth sites seems to be independent of normal chromosome replication, these results confirm previous studies showing that chromosome replication is necessary for the terminal events of growth site development which result in the division of a site into two separate poles. Two classes of models for the regulation of growth site initiation are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Vicente M. Cell length, cell growth and cell division. Nature. 1976 Nov 25;264(5584):328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- Edelstein E. M., Rosenzweig M. S., Daneo-Moore L., Higgins M. L. Unit cell hypothesis for Streptococcus faecalis. J Bacteriol. 1980 Jul;143(1):499–505. doi: 10.1128/jb.143.1.499-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. W., Daneo-Moore L., Higgins M. L. Initiation of wall assembly sites in Streptococcus faecium. J Bacteriol. 1983 May;154(2):573–579. doi: 10.1128/jb.154.2.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. Cell division during inhibition of deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1968 May;95(5):1627–1633. doi: 10.1128/jb.95.5.1627-1633.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Approximation of the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1188–1191. doi: 10.1128/jb.134.3.1188-1191.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Cellular autolytic activity in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Feb;133(2):822–829. doi: 10.1128/jb.133.2.822-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Gen Microbiol. 1981 Mar;123(1):151–161. doi: 10.1099/00221287-123-1-151. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Control of cell growth in bacteria: experiments with thymine starvation. J Bacteriol. 1971 Feb;105(2):472–476. doi: 10.1128/jb.105.2.472-476.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., ISZARD L., ROGERS N. B., SHOCKMAN G. D. Cell multiplication studied with an electronic particle counter. J Bacteriol. 1961 Dec;82:857–866. doi: 10.1128/jb.82.6.857-866.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]




