Abstract
Amorphous, unstained, frozen-hydrated sections of bacteria provide a faithful high-resolution image of procaryotic cells. Conventional preparation artifacts due to fixation, staining, and dehydration are nonexistent. Freezing damage is avoided by using glucose as a cryoprotectant. Cutting damage on frozen material is severe, but sectioning artifacts, being always related to the cutting direction, can be systematically recognized and thus taken into consideration. Geometry and density distribution of the bacterial envelope can be resolved to about 3 nm. The following main features have been observed. In Escherichia coli the inner and outer membranes have an approximately uniform density profile. The distance between the two membranes is constant, ca. 33 nm. In Staphylococcus aureus the cell wall is ca. 40 nm wide. It is bordered on the cytoplasmic side by an asymmetric 5.5-nm-wide bilayer. The bacterial nucleoid, clearly visible with conventional preparation methods, appears in exponentially growing bacteria as an ill-defined central region with approximately the same density as the rest of the cytoplasm. It becomes more clearly visible when bacteria are in the stationary phase, plasmolysed, fixed, or stained. We confirm that "mesosomes," hitherto quite often considered to be essential organelles in all procaryotes, are artifacts. They appear in large numbers during osmium fixation.
Full text
PDF




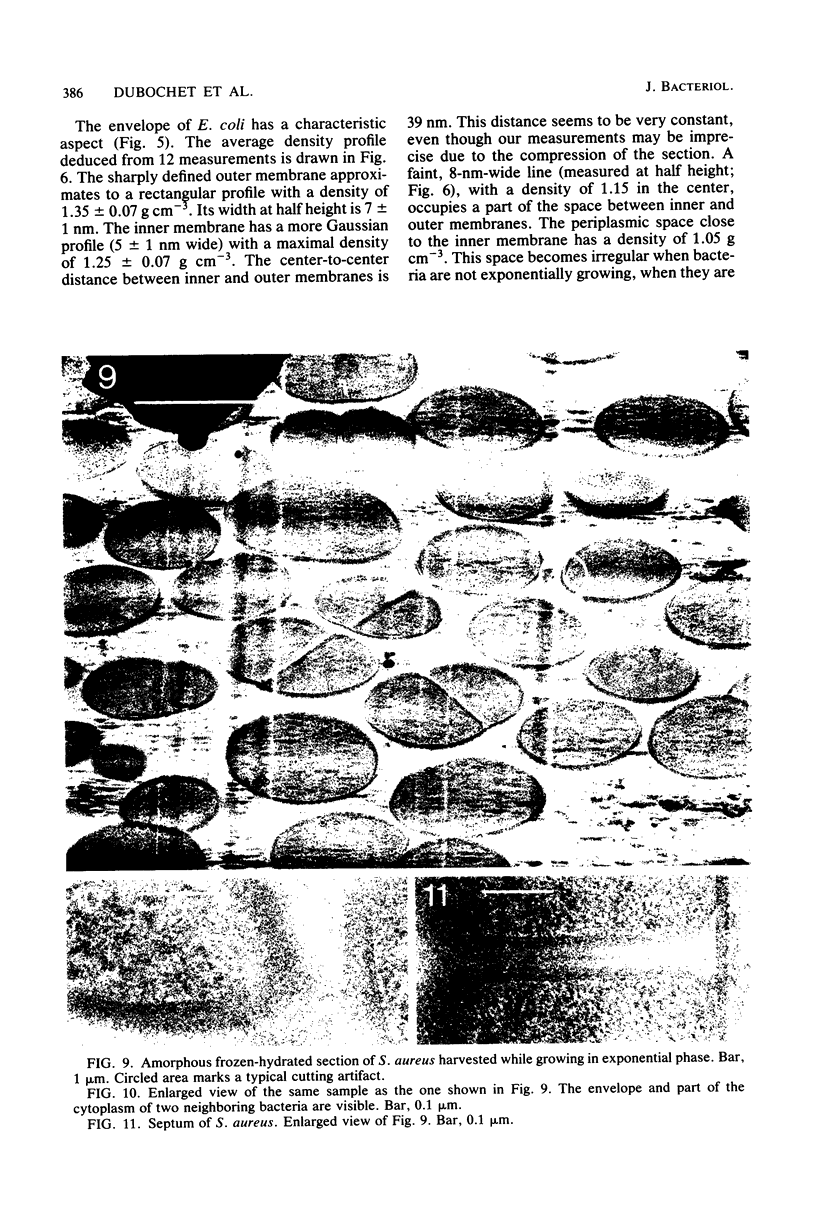
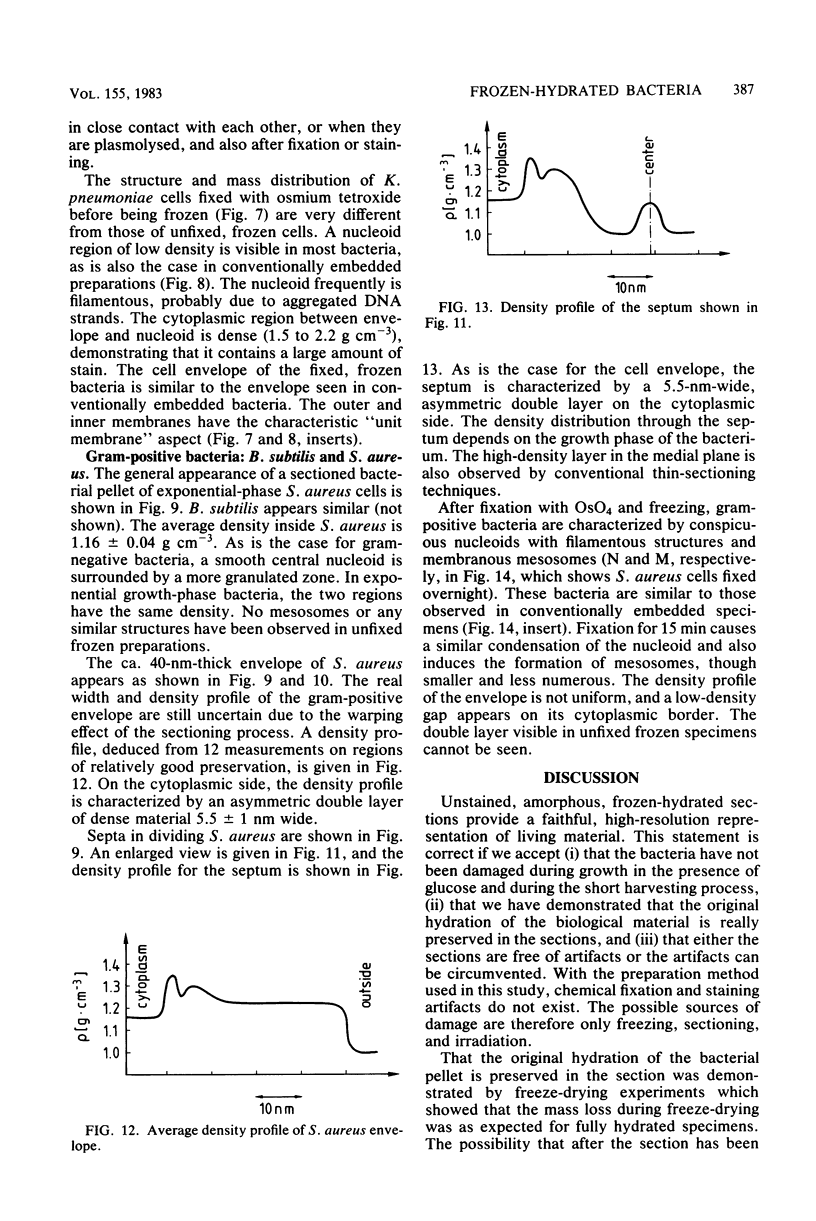
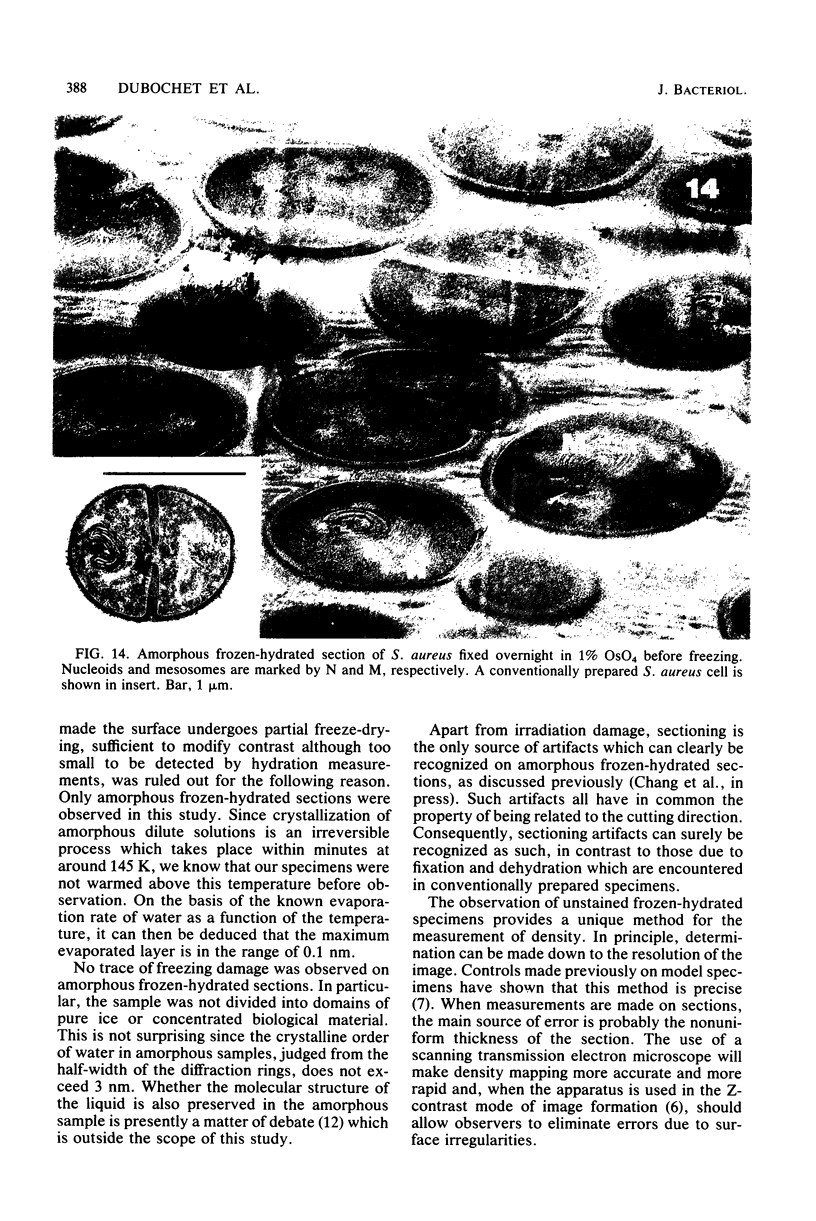


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- Carlemalm E., Kellenberger E. The reproducible observation of unstained embedded cellular material in thin sections: visualisation of an integral membrane protein by a new mode of imaging for STEM. EMBO J. 1982;1(1):63–67. doi: 10.1002/j.1460-2075.1982.tb01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersold H. R., Cordier J. L., Lüthy P. Bacterial mesosomes: method dependent artifacts. Arch Microbiol. 1981 Sep;130(1):19–22. doi: 10.1007/BF00527066. [DOI] [PubMed] [Google Scholar]
- Fooke-Achterrath M., Lickfeld K. G., Reusch V. M., Jr, Aebi U., Tschöpe U., Menge B. Close-to-life preservation of Staphylococcus aureus mesosomes for transmission electron microscopy. J Ultrastruct Res. 1974 Nov;49(2):270–285. doi: 10.1016/s0022-5320(74)80037-7. [DOI] [PubMed] [Google Scholar]
- Hohn B., Hohn T. Activity of empty, headlike particles for packaging of DNA of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2372–2376. doi: 10.1073/pnas.71.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickfeld K. G., Achterrath M., Hentrich F., Kolehmainen-Seveus L., Persson A. Die Feinstrukturen von Pseudomonas aeruginosa in ihrer Deutung durch die Gefrierätztechnik, Ultramikrotomie und Kryo-Ultramikrotomie. J Ultrastruct Res. 1972 Jan;38(1):27–45. doi: 10.1016/s0022-5320(72)90082-2. [DOI] [PubMed] [Google Scholar]
- Lickfeld K. G. Der frostgeätzte Bakterienkern. Ein Beitrag zur Klärung seiner Tertiärstruktur. Z Zellforsch Mikrosk Anat. 1968;88(4):560–564. [PubMed] [Google Scholar]
- Lickfeld K. G., Menge B., Wunderli H., van den Broek J., Kellenberger E. The interpretation and quantitation of sliced intracellular bacteriophages and phage-related particles. J Ultrastruct Res. 1977 Aug;60(2):148–168. doi: 10.1016/s0022-5320(77)80062-2. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]







