Abstract
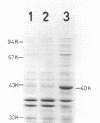
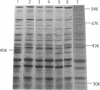
A membrane protein with a molecular weight of 40,000 (40K protein) was induced concurrently with cell filamentation by cyclic AMP (cAMP) in a fic mutant. In the crp mutant and the wild-type strain, cell filamentation by cAMP was not observed, and the 40K protein was not induced. Induction of the 40K protein is regulated by the cAMP-cAMP receptor protein complex and is closely related to cell filamentation by cAMP in the fic mutant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman E. M., Dills S. S., Melton T., Dobrogosz W. J. Cyclic adenosine 3',5'-monophosphate regulation of the bacteriophage T6/colicin K receptor in Escherichia coli. J Bacteriol. 1979 Nov;140(2):369–376. doi: 10.1128/jb.140.2.369-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono R., Yamasaki M., Tamura G. Changes in composition of envelope proteins in adenylate cyclase- or cyclic AMP receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1978 Nov;136(2):812–814. doi: 10.1128/jb.136.2.812-814.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby V., Holland I. B. A kinetic analysis of cell division, and induction and stability of recA protein in U.V. Irradiated ion+ and ion-strains of Escherichia coli K12. Mol Gen Genet. 1979 Oct 2;176(1):121–128. doi: 10.1007/BF00334303. [DOI] [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Nucleotide sequence of the lexA gene of E. coli. Cell. 1981 Mar;23(3):689–697. doi: 10.1016/0092-8674(81)90432-3. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Moore B. A., Masters M., Donachie W. D. Individual proteins are synthesized continuously throughout the Escherichia coli cell cycle. J Bacteriol. 1979 May;138(2):352–360. doi: 10.1128/jb.138.2.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Pardee A. B. Inhibition of Escherichia coli division by protein X. J Bacteriol. 1978 Mar;133(3):1492–1500. doi: 10.1128/jb.133.3.1492-1500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti S., Park J. T. Genetic characterization of a filament-forming, lipoprotein-deficient mutant of Escherichia coli. J Bacteriol. 1980 Sep;143(3):1289–1294. doi: 10.1128/jb.143.3.1289-1294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Tanabe H., Nakamoto Y., Kawamukai M., Sakai H., Himeno M., Komano T., Hirota Y. Inhibitory effect of adenosine 3',5'-phosphate on cell division of Escherichia coli K-12 mutant derivatives. J Bacteriol. 1981 Sep;147(3):1105–1109. doi: 10.1128/jb.147.3.1105-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]