Abstract
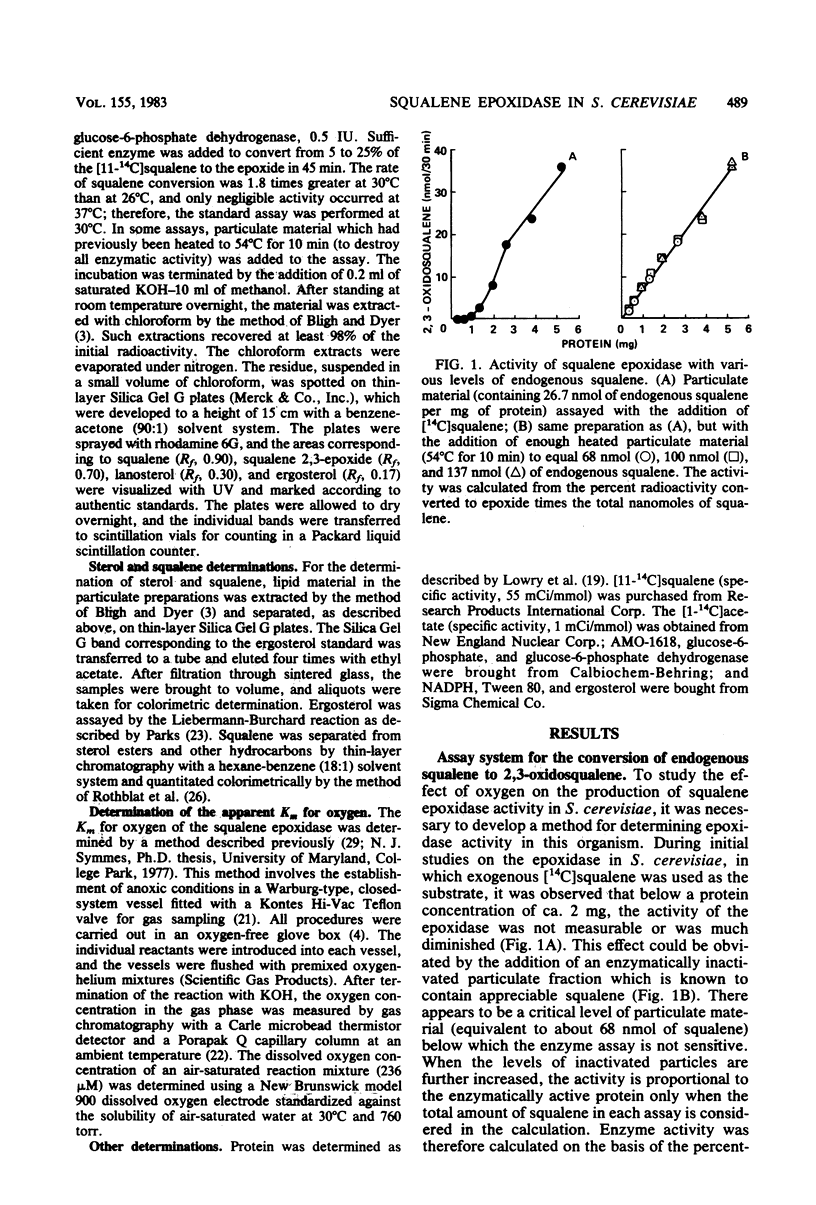
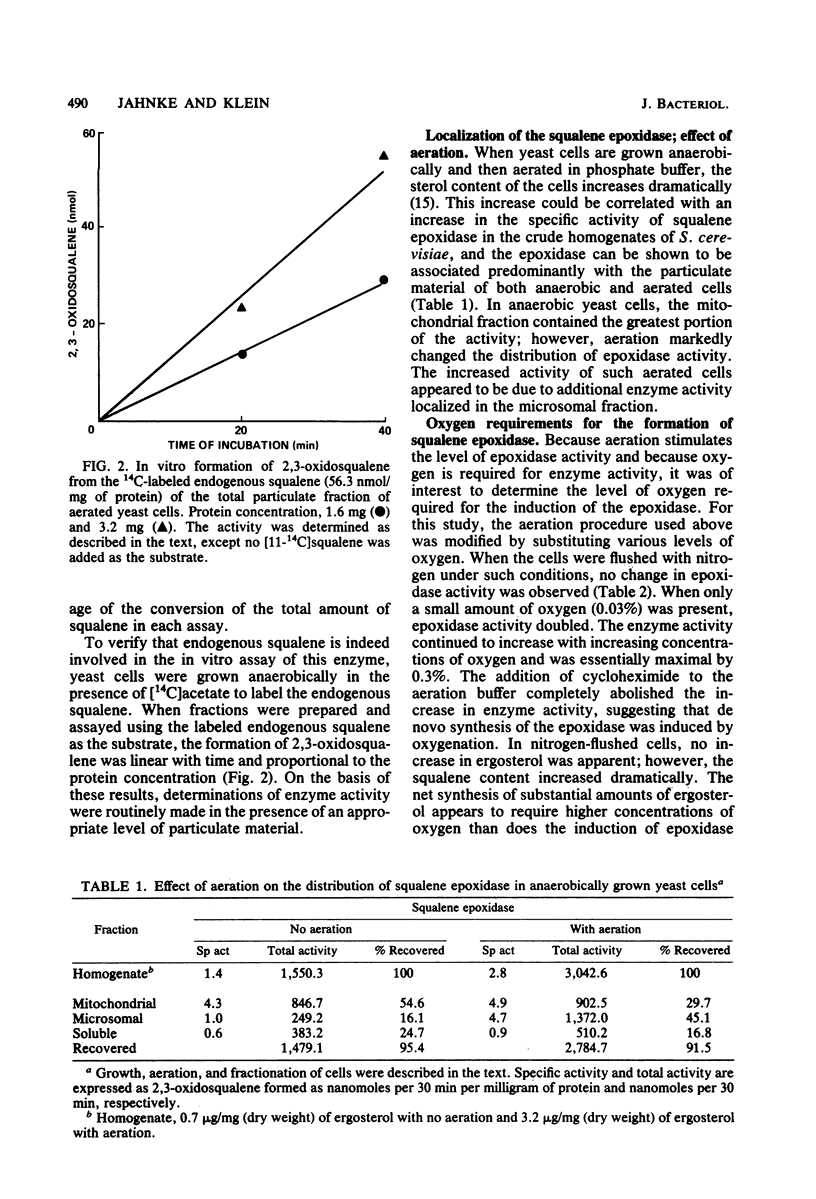
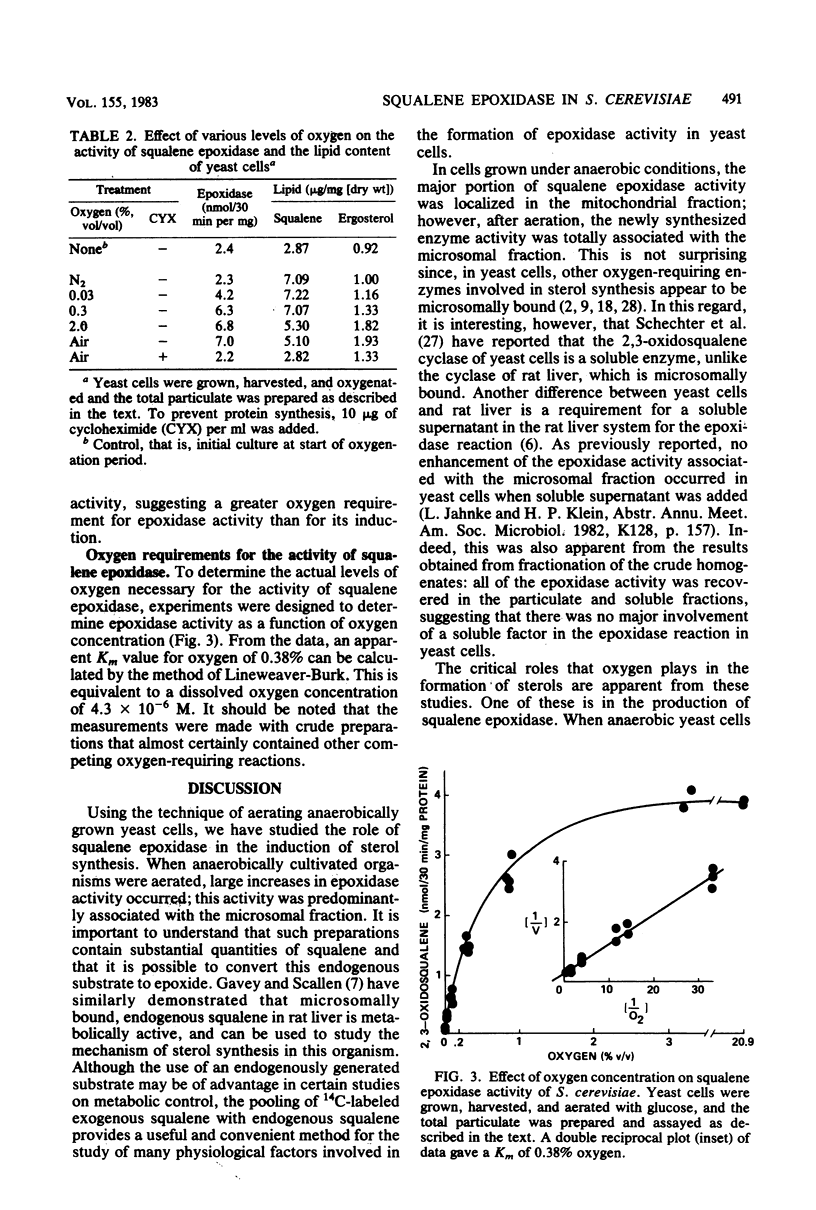
The effect of oxygen on squalene epoxidase activity in Saccharomyces cerevisiae was investigated. In cells grown in standing cultures, the epoxidase was localized mainly in the "mitochondrial" fraction. Upon aeration, enzyme activity increased and the newly formed enzyme was associated with the "microsomal" fraction. At 0.03% (vol/vol) oxygen, epoxidase levels doubled, whereas the ergosterol level was only slightly increased. Cycloheximide inhibited the increase in epoxidase under these conditions. An apparent Km for oxygen of 0.38% (vol/vol) was determined from a crude particulate preparation for the epoxidase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Yoshida Y., Sato R., Susani M., Ruis H. Involvement of cytochrome b5 and a cyanide-sensitive monooxygenase in the 4-demethylation of 4,4-dimethylzymosterol by yeast microsomes. Biochim Biophys Acta. 1981 Jan 26;663(1):194–202. doi: 10.1016/0005-2760(81)90205-8. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cox M. E., Mangels J. I. Improved chamber for the isolation of anaerobic microorganisms. J Clin Microbiol. 1976 Jul;4(1):40–45. doi: 10.1128/jcm.4.1.40-45.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T. J., Paleg L. G. Inhibition of Sterol Biosynthesis by 2-Isopropyl-4-dimethylamino-5-methylphenyl-1-piperidine Carboxylate Methyl Chloride in Tobacco and Rat Liver Preparations. Plant Physiol. 1972 Mar;49(3):417–420. doi: 10.1104/pp.49.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. B., Bloch K. Purification and properties of a soluble protein activator of rat liver squalene epoxidase. J Biol Chem. 1977 Aug 10;252(15):5381–5385. [PubMed] [Google Scholar]
- Gavey K. L., Scallen T. J. Studies on the conversion of enzymatically generated, microsome-bound squalene to sterol. J Biol Chem. 1978 Aug 10;253(15):5476–5483. [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Hata S., Nishino T., Komori M., Katsuki H. Involvement of cytochrome P-450 in delta 22-desaturation in ergosterol biosynthesis of yeast. Biochem Biophys Res Commun. 1981 Nov 16;103(1):272–277. doi: 10.1016/0006-291x(81)91689-2. [DOI] [PubMed] [Google Scholar]
- Jahnke L., Klein H. P. Oxygen as a factor in eukaryote evolution: some effects of low levels of oxygen on Saccharomyces cerevisiae. Orig Life. 1979 Sep;9(4):329–334. doi: 10.1007/BF00926825. [DOI] [PubMed] [Google Scholar]
- Johnson B., Nelson S. J., Brown C. M. Influence of glucose concentration on the physiology and lipid composition of some yeasts. Antonie Van Leeuwenhoek. 1972;38(2):129–136. doi: 10.1007/BF02328084. [DOI] [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN H. P., EATON N. R., MURPHY J. C. Net synthesis of sterols in resting cells of Saccharomyces cerevisiae. Biochim Biophys Acta. 1954 Apr;13(4):591–591. doi: 10.1016/0006-3002(54)90382-0. [DOI] [PubMed] [Google Scholar]
- KLEIN H. P. Synthesis of lipids in resting cells of Saccharomyces cerevisiae. J Bacteriol. 1955 Jun;69(6):620–627. doi: 10.1128/jb.69.6.620-627.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. P., Jahnke L. Cellular localization of acetyl-coenzyme A synthetase in yeast. J Bacteriol. 1968 Nov;96(5):1632–1639. doi: 10.1128/jb.96.5.1632-1639.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. P., Jahnke L. Variations in the localization of acetyl-coenzyme A synthetase in aerobic yeast cells. J Bacteriol. 1971 May;106(2):596–602. doi: 10.1128/jb.106.2.596-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. P. Nature of Particles Involved in Lipid Synthesis in Yeast. J Bacteriol. 1965 Jul;90(1):227–234. doi: 10.1128/jb.90.1.227-234.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. P., Volkmann C. M., Leaffer M. A. Subcellular sites involved in lipid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1967 Jul;94(1):61–65. doi: 10.1128/jb.94.1.61-65.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munoz E. F., Silverman M. P. Gas-tight flask for the concurrent measurement of gas metabolism and growth in methane-oxidizing bacteria. Appl Microbiol. 1974 Sep;28(3):507–509. doi: 10.1128/am.28.3.507-509.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. W. Metabolism of sterols in yeast. CRC Crit Rev Microbiol. 1978;6(4):301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- ROTHBLAT G. H., MARTAK D. S., KRITCHEVSKY D. A quantitative colorimetric assay for squalene. Anal Biochem. 1962 Jul;4:52–56. doi: 10.1016/0003-2697(62)90019-2. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Mitochondrial and peroxisomal contributions to the energy metabolism of Saccharomyces cerevisiae in continuous culture. J Gen Microbiol. 1973 Dec;79(2):205–217. doi: 10.1099/00221287-79-2-205. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Yue S. B., Stewart P. R. Effects of oxygen tension and glucose repression of mitochondrial protein synthesis in continuous cultures of Saccharomyces cerevisiae. J Bacteriol. 1974 May;118(2):523–533. doi: 10.1128/jb.118.2.523-533.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter I., Sweat F. W., Bloch K. Comparative properties of 2,3-oxidosqualene-lanosterol cyclase from yeast and liver. Biochim Biophys Acta. 1970 Dec 16;220(3):463–468. doi: 10.1016/0005-2744(70)90277-9. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., BLOCH K. On the mechanism of enzymatic cyclization of squalene. J Biol Chem. 1957 Jun;226(2):931–939. [PubMed] [Google Scholar]


