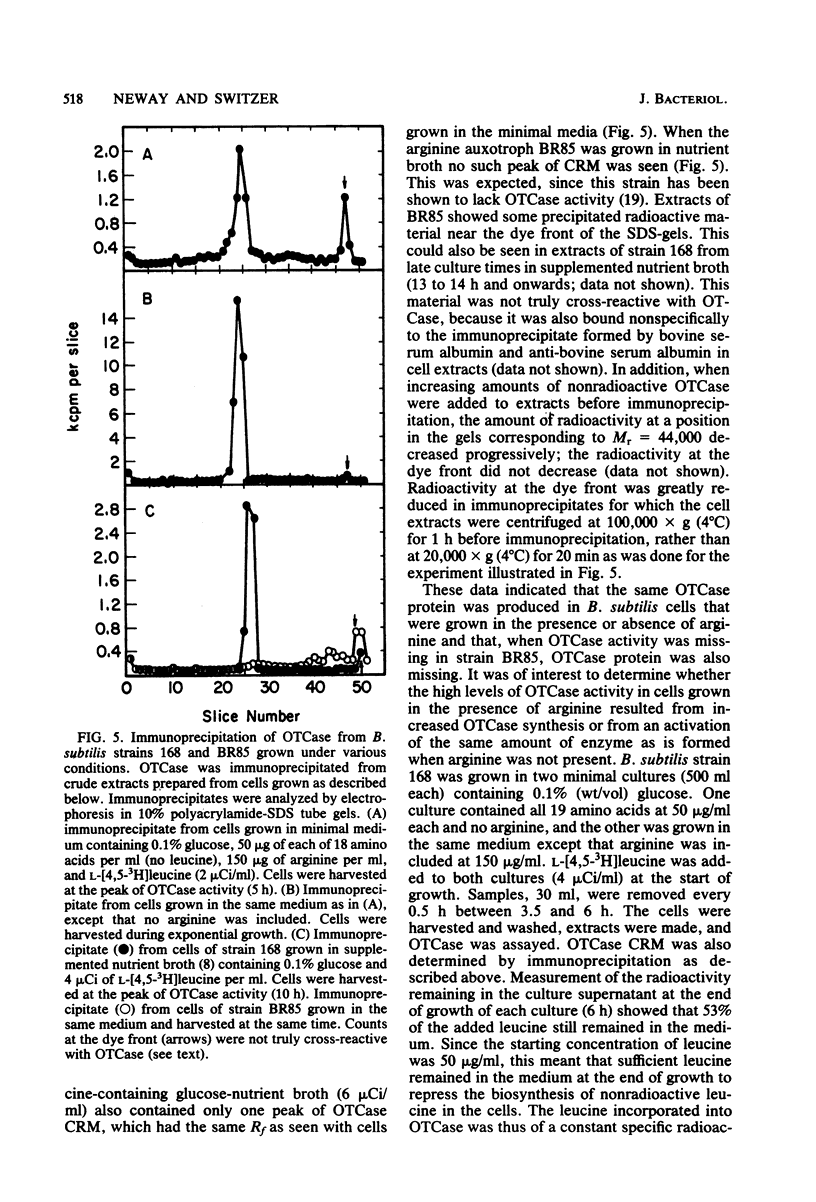
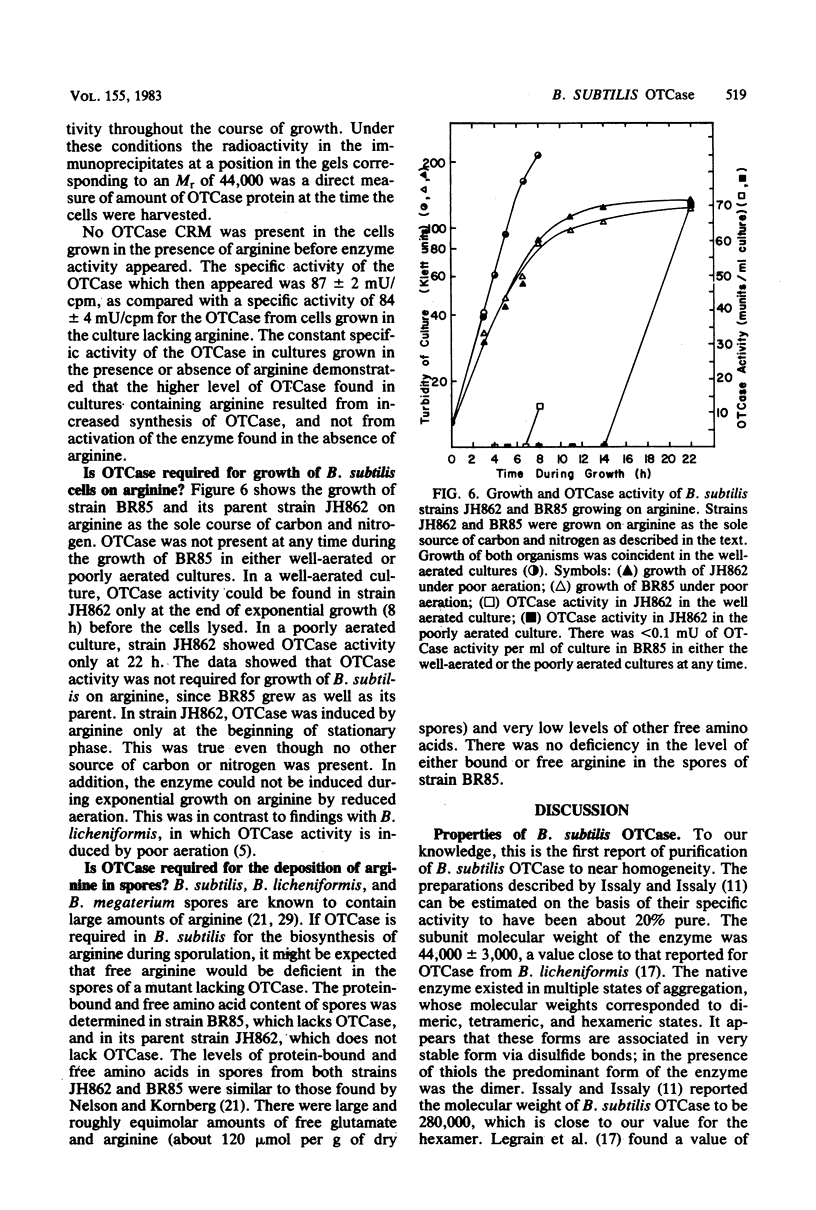
Abstract
A procedure was developed for purification of ornithine transcarbamylase (OTCase) to near homogeneity from Bacillus subtilis 168. The purified native enzyme existed as a mixture of dimeric, tetrameric, and hexameric forms, but was converted to the dimer in the presence of 2-mercaptoethanol. The molecular weight of the subunit was 44,000. Some general kinetic properties of the enzyme were described. OTCase was repressed by arginine in growing B. subtilis cells, but the enzyme was induced by arginine at the end of exponential growth. Specific antibodies against the purified OTCase were used to show that the same enzyme was produced under all conditions. These results and studies of a mutant lacking OTCase demonstrated that B. subtilis produced only a single OTCase. OTCase was clearly required for arginine biosynthesis, but the physiological function of OTCase induction by arginine was obscure. OTCase was not induced by, or required for, growth on arginine as a carbon and nitrogen source. Absence of OTCase in a mutant did not alter the yield or arginine content of its spores in comparison to a strain containing OTCase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Broman K., Lauwers N., Stalon V., Wiame J. M. Oxygen and nitrate in utilization by Bacillus licheniformis of the arginase and arginine deiminase routes of arginine catabolism and other factors affecting their syntheses. J Bacteriol. 1978 Sep;135(3):920–927. doi: 10.1128/jb.135.3.920-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman K., Stalon V., Wiame J. M. The duplication of arginine catabolism and the meaning of the two ornithine carbamoyltransferases in Bacillus licheniformis. Biochem Biophys Res Commun. 1975 Sep 16;66(2):821–827. doi: 10.1016/0006-291x(75)90583-5. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 8. Patterns of enzyme development during growth and sporulation of Baccillus subtilis. J Biol Chem. 1968 Sep 25;243(18):4653–4660. [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J. Synthesis and properties of delta-N-(phosphonacetyl)-L-ornithine. A transition-state analog inhibitor of ornithine transcarbamylase. Arch Biochem Biophys. 1978 May;188(1):137–144. doi: 10.1016/0003-9861(78)90366-1. [DOI] [PubMed] [Google Scholar]
- Issaly I. M., Issaly A. S. Control of ornithine carbamoyltransferase activityby arginase in Bacillus subtilis. Eur J Biochem. 1974 Dec 2;49(3):485–495. doi: 10.1111/j.1432-1033.1974.tb03853.x. [DOI] [PubMed] [Google Scholar]
- Issaly I. M., Issaly A. S., Reissig J. L. Carbamyl phosphate biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1970 Mar 18;198(3):482–494. doi: 10.1016/0005-2744(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol. 1969 Jan 14;39(1):73–87. doi: 10.1016/0022-2836(69)90334-9. [DOI] [PubMed] [Google Scholar]
- Karlström O., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. II. Application to the physiological evidence. J Mol Biol. 1969 Jan 14;39(1):89–94. doi: 10.1016/0022-2836(69)90335-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laishley E. J., Bernlohr R. W. The regulation and kinetics of the two ornithine transcarbamylase enzymes of Bacillus licheniformis. Biochim Biophys Acta. 1968 Nov 19;167(3):547–554. doi: 10.1016/0005-2744(68)90044-2. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Noullez J. P., Mercenier A., Simon J. P., Broman K., Wiame J. M. Structure and function of ornithine carbamoyltransferases. Eur J Biochem. 1977 Nov 1;80(2):401–409. doi: 10.1111/j.1432-1033.1977.tb11895.x. [DOI] [PubMed] [Google Scholar]
- MAHLER I., NEUMANN I. M., MARMUR J. Studies of genetic-units controlling arginine biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1963 May 28;72:69–79. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Neway J. O., Switzer R. L. Degradation of ornithine transcarbamylase in sporulating Bacillus subtilis cells. J Bacteriol. 1983 Aug;155(2):522–530. doi: 10.1128/jb.155.2.522-530.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Characterization of pyrimidine-repressible and arginine-repressible carbamyl phosphate synthetases from Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):82–91. doi: 10.1128/jb.137.1.82-91.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Synthesis and inactivation of carbamyl phosphate synthetase isozymes of Bacillus subtilis during growth and sporulation. J Bacteriol. 1979 Dec;140(3):769–773. doi: 10.1128/jb.140.3.769-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Shindler D. B., Prescott L. M. Improvements on the Prescott-Jones method for the colorimetric analysis of ureido compounds. Anal Biochem. 1979 Sep 1;97(2):421–422. doi: 10.1016/0003-2697(79)90096-4. [DOI] [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. The occurrence of a catabolic and an anabolic ornithine carbamoyltransferase in Pseudomonas. Biochim Biophys Acta. 1967 May 16;139(1):91–97. doi: 10.1016/0005-2744(67)90115-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]