Abstract
We have used mice in which the gene for cytosolic phospholipase A2 (cPLA2) has been disrupted to demonstrate the absolute requirement for cPLA2 in both the immediate and the delayed phases of eicosanoid generation by bone marrow-derived mast cells. For the immediate phase, quantitative analysis of the products of the 5-lipoxygenase pathway showed that gene disruption of cPLA2 prevented the provision of arachidonic acid substrate for biosynthesis of proximal intermediates. By analogy, we conclude that arachidonic acid substrate was also not available to prostaglandin endoperoxide synthase 1 in the immediate phase of prostaglandin (PG) D2 generation. These defects occurred with two distinct stimuli, stem cell factor and IgE/antigen, which were, however, sufficient for signal transduction defined by exocytosis of β-hexosaminidase. Whereas cPLA2 is essential for immediate eicosanoid generation by providing arachidonic acid, its role in delayed-phase PGD2 generation is more complex and involves the activation-dependent induction of prostaglandin endoperoxide synthase 2 and the supply of arachidonic acid for metabolism to PGD2.
Leukotrienes and prostaglandins, derived from the oxidative metabolism of arachidonic acid (1, 2), are potent lipid mediators of tissue inflammation (3, 4). The first step in the generation of these lipid mediators, collectively known as eicosanoids, is the liberation of esterified arachidonic acid from the sn-2 position of cell membrane glycerophospholipids by the action of phospholipase A2 (PLA2) (5). The family of mammalian PLA2 enzymes includes the 85-kDa group IV cytosolic PLA2 (cPLA2); a number of low molecular weight, cysteine-rich PLA2 enzymes, among which are the group IIA and group V PLA2 species; and calcium-independent species of PLA2 (6). The contribution of different PLA2 enzymes, especially cPLA2 as opposed to group IIA and group V PLA2, to the provision of arachidonic acid for eicosanoid generation remains unclear (7–9).
Two phases of eicosanoid generation have been defined in mouse bone marrow-derived mast cells (BMMC) (10, 11) and in the MMC34 mast cell line (12, 13). The immediate phase, elicited by cross-linking the high-affinity Fc receptor for IgE (FcɛRI) or by ligation of c-kit with stem cell factor (SCF), is characterized by the rapid generation of prostaglandin (PG) D2, which depends on the action of constitutively expressed prostaglandin endoperoxide synthase (PGHS) 1, and leukotriene (LT) C4 (12, 14). The delayed phase, elicited by SCF in combination with IL-1β and IL-10 (10) or by antigen activation after sensitization with hapten-specific IgE, with (11) or without (13) cytokine priming, is characterized by the generation of PGD2 in the absence of leukotrienes. Importantly and distinctively, delayed-phase PGD2 generation depends on the induced expression of PGHS-2 (10–14).
The species of PLA2 supplying arachidonic acid in each phase of eicosanoid generation is not resolved. cPLA2 was implicated in the immediate phase by its phosphorylation within 2 min of activation through FcɛRI or c-kit (14) and the inhibition of the immediate response by preincubation of BMMC with methyl arachidonyl fluorophosphate (MAFP), a cPLA2 inhibitor (12). Whereas Reddy and colleagues (15) also implicated group V PLA2 in the immediate generation of PGD2 by inhibition with antisense DNA, we found that scalaradial, a relatively preferential inhibitor of the low molecular weight species of PLA2, did not inhibit the immediate phase of either PGD2 or LTC4 generation (16). By contrast, in our studies, the delayed phase of PGD2 generation was inhibited by heparin, which released a PLA2 activity from the cell into the culture medium, and by scalaradial (16). Although heparin binds strongly to the group IIA PLA2 (17) and has been used as an agent to implicate that enzyme in eicosanoid biosynthesis (18), both immediate and delayed PGD2 generation were intact in BMMC derived from mice in which the gene for group IIA PLA2 was naturally disrupted (15, 16). We therefore concluded that a low molecular weight PLA2 species distinct from the group IIA enzyme participated in the delayed response. However, Reddy and Herschman (12) demonstrated that the delayed phase of PGD2 generation, like the immediate, was inhibited by MAFP, thereby implicating cPLA2 in both phases.
To clarify the role of cPLA2 in the immediate and the delayed phases of eicosanoid generation in the mast cell, we turned from pharmacologic approaches to the analysis of BMMC derived from mice in which the gene for cPLA2 has been disrupted (cPLA2−/−) (19). We now demonstrate that although cPLA2 is required for both the immediate and the delayed phases of eicosanoid generation, its role in the delayed phase is complex. The observation that BMMC derived from cPLA2−/− mice respond to treatment with cytokines and exogenous arachidonic acid with induction of PGHS-2 and delayed generation of PGD2, but do not respond to treatment with cytokines alone, reveals a dual role for cPLA2. That role includes both the amplification of the induction of PGHS-2 and the supply of arachidonic acid as substrate to PGHS-2 in the delayed phase of PGD2 generation. In the immediate phase, cPLA2 is required to provide substrate to both 5-lipoxygenase (5-LO) and PGHS-1.
MATERIALS AND METHODS
Materials.
Mouse recombinant IL-1β (Genzyme), WEHI-3 cells (American Type Culture Collection), arachidonic acid, and PGE2 (Cayman Chemicals, Ann Arbor, MI) were purchased. Mouse recombinant IL-3, IL-9, and IL-10 were produced by expression in insect cells, and their concentrations were determined as described (10, 20). William Smith (Michigan State University, East Lansing, MI) supplied rabbit polyclonal antiserum to PGHS-2, and Jim Clark (Genetics Institute, Boston, MA) supplied rabbit polyclonal antiserum to cPLA2.
Culture of BMMC.
Bone marrow cells from cPLA2−/− mice, strain-matched cPLA2+/+ control mice, and BALB/c mice (The Jackson Laboratory) were cultured for 3–7 weeks in 50% enriched medium (RPMI 1640 medium containing 100 units/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamycin, 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 10% fetal calf serum)/50% WEHI-3 cell-conditioned medium as described (21).
Activation of BMMC.
To assess IgE- and SCF-dependent immediate eicosanoid generation, BMMC were resuspended at a concentration of 107 cells per ml in 50% WEHI-3 cell-conditioned medium and sensitized with a 1:100 dilution of monoclonal mouse IgE anti-trinitrophenyl (TNP) ascites at 37°C overnight (conditions for optimal sensitization of BMMC for FcɛRI-dependent activation). The cells were washed in enriched medium, resuspended at a concentration of 5 × 106 cells per ml in enriched medium, and stimulated at 37°C in a dose-dependent manner with 1–300 ng/ml TNP-BSA to establish an optimal concentration or with 100 ng/ml SCF for 15 min at 37°C. The reaction was stopped by centrifugation of cells at 120 × g at 4°C for 5 min, and the supernatants were retained for assay of PGD2 generation and percentage of β-hexosaminidase release (21, 22). For reverse-phase HPLC analysis of leukotriene generation the same protocol was followed except that BMMC were activated at a concentration of 107 cells per ml.
To assess cytokine-dependent delayed-phase generation of PGD2, BMMC were resuspended at a concentration of 106 cells per ml in enriched medium containing 100 ng/ml SCF, 10 units/ml IL-10, and 5 ng/ml IL-1β (10). After 1 h of culture at 37°C, the cells were centrifuged at 200 × g for 5 min to remove the products of the immediate phase of eicosanoid generation and resuspended in fresh medium with fresh cytokines. The cells were then cultured for an additional 7 h and centrifuged at 200 × g for 5 min at 4°C. The cell pellets were retained for SDS/PAGE immunoblot analysis and the supernatants for assay of PGD2. In selected experiments, arachidonic acid or PGE2 was added to BMMC in a dose-dependent manner.
β-Hexosaminidase was quantitated by spectrophotometric analysis of the hydrolysis of p-nitrophenyl-β-d-2-acetamido-2-deoxyglucopyranoside (22). PGD2 was measured by using RIA (Amersham Pharmacia). Leukotriene release in the immediate phase was measured by using reverse-phase HPLC (23).
SDS/PAGE Immunoblot Analysis.
The expression of cPLA2 and PGHS-2 was analyzed by SDS/PAGE immunoblot with rabbit polyclonal antisera at 1:3,000 and 1:5,000 dilutions, respectively (10, 20). Proteins were visualized with an enhanced chemiluminescence detection system (Pierce). The induced expression of PGHS-2 in BMMC from cPLA2+/+ mice and cPLA2−/− mice was quantitated by densitometry with imagequant software.
Data Analysis.
Each experiment was performed at least three times. Pooled data are expressed as mean ± SEM and were analyzed by Student’s t test for unpaired data.
RESULTS
Development of BMMC.
By 4 weeks of culture with WEHI-3 cell-conditioned medium as the source of IL-3, >98% of cells derived from both cPLA2−/− and cPLA2+/+ mice were mast cells as indicated by metachromatic staining with toluidine blue. However, significantly more BMMC were obtained from cPLA2−/− mice than from cPLA2+/+ mice. From 107 starting bone marrow cells, 5.9 ± 1.5 × 106 BMMC and 1.3 ± 0.2 × 106 BMMC were obtained at 3 weeks from cPLA2−/− mice and cPLA2+/+ mice, respectively (n = 9, P = 0.008). The absence of cPLA2 in BMMC derived from cPLA2−/− mice was confirmed by using SDS/PAGE immunoblot analysis. A ≈110-kDa protein was detected by antiserum to cPLA2 in extracts of BMMC derived from cPLA2+/+ and BALB/c mice but not in extracts of BMMC derived from cPLA2−/− mice (Fig. 1).
Figure 1.
Expression of cPLA2 in BMMC. SDS/PAGE immunoblot analysis of the expression of cPLA2 in BMMC derived from a BALB/c mouse, three cPLA2+/+ control mice, and three cPLA2−/− mice. Extracts from 2 × 105 cells were applied to each lane.
Immediate-Phase Mediator Generation.
Immediate-phase mediator generation in response to antigen stimulation of IgE-sensitized BMMC was evaluated in BMMC derived from 9 BALB/c mice on 9 occasions, in BMMC from 7 cPLA2+/+ mice on 11 occasions, and in BMMC from 9 cPLA2−/− mice on 17 occasions. β-Hexosaminidase, PGD2, and leukotriene products were released by cPLA2+/+ BMMC, and β-hexosaminidase was released by cPLA2−/− BMMC in a dose-dependent manner in response to TNP-BSA after sensitization with IgE anti-TNP. Maximal mediator generation occurred in response to 100 ng/ml TNP-BSA, consistent with previous data (14).
BMMC from BALB/c, cPLA2+/+, and cPLA2−/− mice released 16.5 ± 3.1%, 19.0 ± 5.2%, and 30.4 ± 3.5% β-hexosaminidase, respectively, 15 min after activation with 100 ng/ml TNP-BSA (Fig. 2A); the release by BMMC from cPLA2−/− mice was significantly greater than the release by BMMC from cPLA2+/+ mice (P = 0.03) and BALB/c mice (P = 0.008). In contrast, PGD2 generation was undetectable in BMMC from cPLA2−/− mice, although BMMC from BALB/c mice and cPLA2+/+ mice generated 2.4 ± 0.8 ng of PGD2 per 106 cells and 3.8 ± 0.7 ng of PGD2 per 106 cells, respectively, in response to 100 ng/ml TNP-BSA (Fig. 2B) (P = 0.0001). Similarly, the generation of LTC4 and other 5-LO pathway metabolites was undetectable in IgE-sensitized BMMC from cPLA2−/− mice in response to antigen even though the response for each metabolite and for their sum appeared to be augmented in BMMC from strain-matched cPLA2+/+ mice compared with BMMC derived from BALB/c mice (Table 1).
Figure 2.
Immediate-phase β-hexosaminidase release and PGD2 generation. Immediate β-hexosaminidase release (%) (A and C) and PGD2 generation (ng per 106 BMMC) (B and D) were measured in BMMC derived from BALB/c, cPLA2+/+, and cPLA2−/− mice sensitized with IgE anti-TNP and activated with either TNP-BSA (IgE/Ag; A and B) or SCF (C and D) for 10 min.
Table 1.
Generation of leukotrienes and pathway intermediates in BMMC derived from BALB/c, cPLA2+/+, and cPLA2−/− mice
| Stimulus | Product | BMMC
|
||
|---|---|---|---|---|
| BALB/c (n = 5) | cPLA2+/+ (n = 3) | cPLA2−/−(n = 3)* | ||
| IgE/Ag | LTC4 | 16.4 ± 4.0 | 73.6 ± 4.0 | 0 |
| LTB4 | 8.0 ± 2.8 | 17.0 ± 2.3 | 0 | |
| 5-HETE | 8.7 ± 3.0 | 34.4 ± 2.0 | 0 | |
| 6-t-LTB4 | 5.0 ± 2.9 | 30.6 ± 3.2 | 0 | |
| SCF | LTC4 | 11.6 ± 4.8 | 14.5 ± 0.7 | 0 |
| LTB4 | 7.0 ± 1.9 | 11.3 ± 1.0 | 0 | |
| 5-HETE | 9.0 ± 3.5 | 1.9 ± .01 | 0 | |
| 6-t-LTB4 | 4.7 ± 2.6 | 3.7 ± 1.7 | 0 | |
Leukotriene (LT) pathway products (ng per 106 cells) were measured by using RP-HPLC in supernatants of BMMC sensitized with IgE anti-TNP and activated with 100 ng/ml TNP-BSA (IgE/Ag) or with 100 ng/ml SCF. 5-HETE, 5-hydroxyeicosatetraenoic acid; 6-t-LTB4, 6-trans-LTB4 diastereoisomers. ∗, P < 0.01 compared to total leukotriene generation from cPLA2+/+ BMMC for both agonists.
Immediate-phase mediator generation in response to 100 ng/ml SCF was evaluated in BMMC derived from 7 BALB/c mice on 7 occasions, in BMMC from 6 cPLA2+/+ mice on 7 occasions, and in BMMC from 7 cPLA2−/− mice on 7 occasions. BMMC from BALB/c, cPLA2+/+, and cPLA2−/− mice released 11.6 ± 3.0%, 6.1 ± 1.5%, and 23.6 ± 7.7% β-hexosaminidase, respectively, 15 min after activation with 100 ng/ml SCF (Fig. 2C). Secretory granule exocytosis in response to SCF was significantly greater in the BMMC derived from cPLA2−/− mice than in BMMC derived from cPLA2+/+ mice (P = 0.04). In contrast, PGD2 generation was undetectable in BMMC from cPLA2−/− mice, although BMMC from BALB/c and cPLA2+/+ mice generated 0.7 ± 0.3 ng PGD2 per 106 cells (P = 0.0001) and 0.8 ± 0.1 ng PGD2 per 106 cells (P = 0.004), respectively, in response to 100 ng/ml SCF (Fig. 2D). Similarly, the generation of LTC4 and other lipoxygenase metabolites was undetectable in BMMC from cPLA2−/− mice in response to SCF (Table 1).
Delayed-Phase PGD2 Generation.
The PGHS-2-dependent delayed-phase generation of PGD2 in response to SCF + IL-10 + IL-1β was determined in BMMC from 5 BALB/c mice on 5 occasions, in BMMC from 6 cPLA2+/+ mice on 6 occasions, and in BMMC from 7 cPLA2−/− mice on 11 occasions. To eliminate the contribution of immediate-phase PGD2 generation by PGHS-1, BMMC were washed 1 h after activation, resuspended in fresh medium and cytokines, and examined for PGD2 generation 7 h later. Delayed-phase PGD2 generation was undetectable in BMMC derived from cPLA2−/− mice. In comparison, BMMC from BALB/c and cPLA2+/+ mice released 3.8 ± 0.8 ng of PGD2 per 106 cells and 2.0 ± 0.4 ng of PGD2 per 106 cells, respectively, 8 h after activation with SCF + IL-10 + IL-1β (P < 0.0001) (Fig. 3A). Importantly, the induced expression of PGHS-2 in BMMC from cPLA2−/− mice was 36.4 ± 6.4% that observed in BMMC from cPLA2+/+ mice 8 h after cytokine-dependent activation (Fig. 3B).
Figure 3.
Delayed-phase PGD2 generation. Delayed-phase PGD2 generation (A) was determined in BMMC derived from BALB/c, cPLA2+/+, and cPLA2−/− mice. BMMC were stimulated with SCF + IL-10 + IL-1β for 1 h, washed, and incubated for 7 h more with the same cytokines. PGD2 released into the supernatant was measured by using RIA. PGHS-2 induction (B) was assessed by SDS/PAGE immunoblotting 8 h after the initial activation with SCF + IL-10 + IL-1β (+) or after continued culture in WEHI-3 cell-conditioned medium (−).
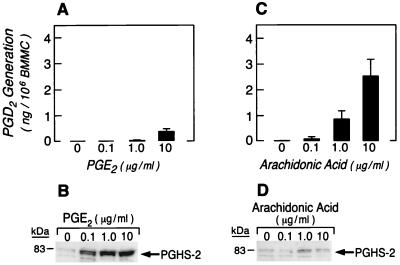
These data indicate that cPLA2 may contribute to delayed-phase PGD2 generation by supplying arachidonic acid to PGHS-2 and/or by facilitating the induction of PGHS-2. Although PGE2 is not an eicosanoid product of BMMC, it was reported to induce PGHS-2 in the MC3T3-E1 mouse osteoblast cell line (24). To investigate the possible roles of cPLA2 both in inducing PGHS-2 and in supplying arachidonic acid to the induced enzyme, the dose-dependent effects of exogenous arachidonic acid and PGE2 on the induction of PGHS-2 and delayed-phase PGD2 generation were assessed in BMMC from cPLA2−/− mice. Arachidonic acid (0.1–10 μg/ml) or PGE2 (0.1–10 μg/ml) was added to the culture medium at the time of cytokine stimulation. To eliminate the contribution of immediate-phase PGD2 generation by PGHS-1, BMMC were washed 1 h after activation as described above and were resuspended in fresh medium containing fresh cytokines and either arachidonic acid or PGE2. The addition of PGE2 to the culture medium led to a marked increase in PGHS-2 even at 0.1 μg/ml, in BMMC from cPLA2−/− mice (Fig. 4B). However, the increment in PGHS-2 was accompanied by only minimal delayed-phase PGD2 generation of 0.3 ± 0.1 ng PGD2 per 106 cells 8 h after cytokine stimulation at the highest PGE2 concentration of 10 μg/ml (Fig. 4A). Therefore, the lack of delayed-phase PGD2 generation in BMMC from cPLA2−/− mice was not due simply to impaired induction of PGHS-2, suggesting that cPLA2 also is needed to provide arachidonic acid to the induced PGHS-2 in the delayed phase. Consistent with this suggestion, stimulation with SCF + IL-10 + IL-1β in the presence of arachidonic acid led to a concentration-dependent restoration of delayed-phase PGD2 generation (Fig. 4C) in BMMC derived from cPLA2−/− mice accompanied by a modest and plateau increase in the expression of PGHS-2 (Fig. 4D). At a concentration of 10 μg/ml arachidonic acid, delayed-phase PGD2 generation reached 2.5 ± 0.7 ng of PGD2 per 106 cells (P = 0.001; n = 9), almost an order of magnitude more than the amount obtained with 10 μg/ml PGE2 (n = 6; P = 0.0001), even though induction of PGHS-2 was less.
Figure 4.
Delayed-phase PGD2 generation and induction of PGHS-2 in the presence of arachidonic acid or PGE2. BMMC were stimulated with SCF + IL-10 + IL-1β in the presence of increasing concentrations of PGE2 or arachidonic acid for 1 h, washed, and incubated for 7 h more with cytokines and PGE2 or arachidonic acid to assess delayed-phase PGD2 generation by RIA (A and C) and PGHS-2 induction by SDS/PAGE immunoblotting (B and D).
DISCUSSION
We have demonstrated in BMMC from mice with disruption of the cPLA2 gene that cPLA2 is absolutely required for immediate-phase generation of the leukotrienes, LTC4 and LTB4, and the prostanoid, PGD2, in response to signaling through c-kit or FcɛRI. We have further found that cPLA2 is also critical for delayed-phase PGHS-2-dependent PGD2 generation acting to amplify the cytokine-dependent induction of PGHS-2 as well as to supply arachidonic acid to the induced enzyme.
BMMC were derived from cPLA2−/− mice and from cPLA2+/+ mice in WEHI-3 cell-conditioned medium according to established protocols. By 4 weeks of culture, >98% of cells derived from both types of mice were mast cells as indicated by metachromatic staining with toluidine blue. Thus, cPLA2 is not required for normal IL-3-dependent development of BMMC from bone marrow. Furthermore, BMMC from cPLA2−/− mice exhibited intact, or even augmented, signal transduction-dependent exocytosis to perturbation of either c-kit or FcɛRI, indicating that signal transduction through these characteristic mast cell receptors is intact. SDS/PAGE immunoblotting confirmed the presence of cPLA2 in BMMC from cPLA2+/+ mice and its absence in BMMC from cPLA2−/− mice (Fig. 1). PCR analysis confirmed that the gene for the group IIA enzyme was disrupted in BMMC from both cPLA2+/+ and cPLA2−/− mice (data not shown) derived from 129 ES cells on a C57BL/6 background; this is consistent with the observation that the gene for group IIA PLA2 is disrupted in 129 and C57BL/6 mice (16, 25, 26). Reverse transcription–PCR analysis established the presence of transcripts for the group V enzyme in BMMC from both cPLA2+/+ and cPLA2−/− mice (data not shown), consistent with studies in BMMC derived from C57BL/6 and AKJ mice (15).
The immediate phase of both leukotriene and PGD2 generation, in response to SCF or to IgE and antigen, was completely ablated in BMMC derived from cPLA2−/− mice, whereas BMMC from cPLA2+/+ mice provided PGD2 and all of the pathway intermediates of leukotriene generation (Fig. 2 and Table 1). The absence of the intermediates, 5-hydroxyeicosatetraenoic acid (5-HETE; derived from 5-hydroperoxyeicosatetraenoic acid) and 6-trans-LTB4 diastereoisomers (derived by the nonenzymatic hydrolysis of LTA4), in reverse-phase HPLC analyses of products from cPLA2−/− BMMC indicates that the biosynthetic failure is to provide arachidonic acid to 5-LO in concert with 5-LO-activating protein. This results in an absence of LTA4 substrate for the terminal enzymes, LTC4 synthase and LTA4 hydrolase. By analogy, there would also be no arachidonic acid for PGHS-1 to convert to PGH2, the substrate for the terminal enzyme, glutathione-dependent, hematopoietic PGD synthase. These findings are compatible with our earlier studies that showed phosphorylation of cPLA2 (14) and failure of an inhibitor of the low molecular weight PLA2 species to attenuate immediate eicosanoid generation (16).
These data are consistent with the observation that in peritoneal macrophages isolated from cPLA2−/− mice immediate LTC4, LTB4, and PGE2 generation in response to the calcium ionophore A23187 is markedly inhibited (19, 27). However, the inclusion of analyses for the proximal products of 5-LO/5-LO-activating protein metabolism of arachidonic acid pinpoints the disruption to the provision of arachidonic acid per se, thereby eliminating any substrates for the terminal pathway enzymes. These data are in contrast to those obtained by using inhibition approaches with human monocytes in which the 5-LO pathway and prostanoid pathways were segregated, with the former requiring a low molecular weight PLA2 and the latter requiring cPLA2 (28, 29). Marshall and colleagues (29) also demonstrated that the immediate generation of LTC4, but not PGD2, was inhibited in BMMC treated with the low molecular weight PLA2 inhibitor, SB203347, and activated with IgE and antigen. The uncoupling of leukotriene and prostanoid pathways for the human monocyte and mouse BMMC (28, 29), but not mouse peritoneal macrophages (19, 27) or mouse BMMC from cPLA2−/− mice (16) (Table 1, Fig. 2), could reflect a lack of specificity of inhibition approaches or a difference in experimental design. Our current studies with BMMC (Table 1), like those with mouse peritoneal macrophages (19, 27), reveal an absolute requirement for cPLA2 in the immediate generation of both prostanoid and leukotriene pathway products.
Reddy and Herschman (12) demonstrated that treatment of BMMC with MAFP, an inhibitor of cPLA2, 10 min before (but not at the time of) activation through FcɛRI inhibited immediate PGD2 generation. LTC4 generation was not examined. In contrast to the findings of Marshall and colleagues (29), they also demonstrated inhibition of immediate phase PGD2 generation in BMMC through the action of SB203347 (12) and in the MMC-34 mast cell line through antisense inhibition of the group V PLA2 (15). Our data indicate an absolute requirement for cPLA2 in the immediate phase of generation of both PGD2 and LTC4 and reveal that group V PLA2 alone is not sufficient. The data from Reddy and colleagues could indicate a cooperative and/or amplifying action of group V PLA2 in the immediate phase of PGD2 generation or may reflect the lack of specificity of pharmacologic and antisense approaches. A cooperative action of cPLA2 with the group V and group IIA low molecular weight enzymes has also been shown in transfected 293S cells and CHO cells (30), which have a small basal expression of cPLA2 and release minimal arachidonic acid in response to A23187. When these cell lines were transfected with either the group IIA or the group V PLA2, they released a substantial amount of arachidonic acid in response to A23187 that was inhibited by MAFP, suggesting that endogenous cPLA2 was required for the action of the low molecular weight enzymes. Such cooperation also has been described in P388D1 macrophages primed with lipopolysaccharide for 1 h and stimulated with platelet-activating factor (31) to release intracellular arachidonic acid, which facilitates the action of the group V PLA2 to provide arachidonic acid for conversion to PGE2 via PGHS-2 over 10 min (31–33).
Our studies also show that cPLA2 is required for the delayed phase of PGD2 generation in BMMC (Fig. 3A), apparently both to supply arachidonic acid and to facilitate the ligand-dependent induction of PGHS-2 (Fig. 3B). In previous studies, we have shown that delayed-phase PGD2 generation is deficient in BMMC derived from 129 mice because of failure of induction of PGHS-2 (16). However, in the present series of experiments, delayed-phase generation of PGD2 was intact in BMMC from the littermate controls, no doubt because of the contribution of the C57BL/6 background, although it was somewhat less than in BMMC from BALB/c mice (Fig. 3A). Delayed-phase generation of PGD2 was seen in each set of BMMC from cPLA2+/+ mice and in none of the BMMC from cPLA2−/− mice. Thus, differences in the genetic background are unlikely to account for the absence of delayed-phase PGD2 generation in BMMC from cPLA2−/− mice or the diminished induction of PGHS-2. PGHS-2 expression was restored by the supply of either arachidonic acid or PGE2 (Fig. 4). However, only arachidonic acid restored the delayed phase of PGD2 generation in the presence of membrane signals via SCF + IL-10 + IL-1β. These observations suggest that cPLA2 is important not only for the amplification of PGHS-2 but also in the supply of arachidonic acid in the delayed phase. A role for cPLA2 in augmenting the induced expression of PGHS-2 has been reported in the MC3T3-E1 osteoblast cell line, in which delayed-phase PGE2 generation in response to tumor necrosis factor α and IL-1β depends on cPLA2 and PGHS-2 (24). Inhibitors of cPLA2 or PGHS-2 reduced the expression of both cPLA2 and PGHS-2, and expression of both enzymes was restored by the addition of exogenous arachidonic acid or PGE2.
That a cooperative action of cPLA2 (Fig. 4) with a low molecular weight enzyme (16) is important in delayed-phase PGD2 generation by nontransformed BMMC is supported by transfection studies in other cells. A delayed phase of arachidonic acid release and PGE2 generation in 293 or CHO cells, stimulated by IL-1β and fetal calf serum, depended on PGHS-2, was augmented by transfection with cPLA2, group IIA PLA2, or group V PLA2 (30), and was inhibited by MAFP (30). Because we washed the BMMC 1 h after activation with the cytokine triad, the delayed PGD2 generation associated with PGHS-2 induction and function would require that any participating low molecular weight PLA2 species be firmly retained with the cell. Inasmuch as our BMMC from cPLA2−/− mice were also naturally disrupted for the group IIA PLA2 gene, the current candidate for a low molecular weight enzyme supporting the function of cPLA2 would be group V PLA2. Our previous findings that exogenously added heparin, which binds cationic PLA2, or scalaradial, an inhibitor of low molecular weight PLA2 enzymes, each inhibited delayed-phase PGD2 generation (16) are compatible with a role for two PLA2 enzymes in this pathway. The requirement that exogenous arachidonic acid be supplied with the cytokine triad to cPLA2−/− BMMC containing group V PLA2 implies a concerted interaction to induce PGHS-2 and provide its substrate.
Acknowledgments
We thank Richard P. Goddeau and Chioma Nwankwo for technical assistance. This work was supported by National Institutes of Health Grants HL36110, AI22531, AI31599 (to K.F.A.), DK02493 (to A.S.), MERIT-DK39773, DK38452, NS10828 (to J.B.), and ES06105 (to B.K.L.); by an International Research Grant from the Japan Eye Bank and by the Kowa Life Science Foundation (to H.F.); by a grant from the Arthritis Foundation and a President’s Grant-in-Aid award from the American Academy of Allergy Asthma and Immunology (to C.O.B.); by American Cancer Society Grant RPG-97-001-01-BE and a Burroughs Wellcome Fund Developing Investigator Award (to J.P.A.); and by a grant from the Hyde and Watson Foundation.
ABBREVIATIONS
- 5-LO
5-lipoxygenase
- BMMC
bone marrow-derived mast cells
- cPLA2
cytosolic phospholipase A2
- FcɛRI
high-affinity Fc receptor for IgE
- LT
leukotriene
- MAFP
methyl arachidonyl fluorophosphate
- PG
prostaglandin
- PGHS
prostaglandin endoperoxide synthase
- PLA2
phospholipase A2
- SCF
stem cell factor
- TNP
trinitrophenyl
References
- 1.Samuelsson B. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 2.Smith W L. Am J Physiol. 1992;263:F181–F191. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- 3.Henderson W R. Ann Intern Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Goetzl E J, An S, Smith W L. FASEB J. 1995;9:1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- 5.Dennis E A. J Biol Chem. 1994;269:13057–13061. [PubMed] [Google Scholar]
- 6.Dennis E A. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 7.Clark J D, Lin L L, Kriz R W, Ramesha C S, Sultzman L A, Lin A Y, Milona N, Knopf J L. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 8.Glover S, Bayburt T, Jonas M, Chi E, Gelb M H. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 9.Tischfield J A. J Biol Chem. 1997;272:17247–17250. doi: 10.1074/jbc.272.28.17247. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Matsumoto R, Austen K F, Arm J P. J Biol Chem. 1994;269:22269–22275. [PubMed] [Google Scholar]
- 11.Murakami M, Bingham C O, III, Matsumoto R, Austen K F, Arm J P. J Immunol. 1995;155:4445–4453. [PubMed] [Google Scholar]
- 12.Reddy S T, Herschman H R. J Biol Chem. 1997;272:3231–3237. doi: 10.1074/jbc.272.6.3231. [DOI] [PubMed] [Google Scholar]
- 13.Kawata R, Reddy S T, Wolner B, Herschman H R. J Immunol. 1995;155:818–825. [PubMed] [Google Scholar]
- 14.Murakami M, Austen K F, Arm J P. J Exp Med. 1995;182:197–206. doi: 10.1084/jem.182.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy S T, Winstead M V, Tischfield J A, Herschman H R. J Biol Chem. 1997;272:13591–13596. doi: 10.1074/jbc.272.21.13591. [DOI] [PubMed] [Google Scholar]
- 16.Bingham C O, III, Murakami M, Fujishima H, Hunt J E, Austen K F, Arm J P. J Biol Chem. 1996;42:25936–25944. doi: 10.1074/jbc.271.42.25936. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, Nakatani Y, Kudo I. J Biol Chem. 1996;271:30041–30051. doi: 10.1074/jbc.271.47.30041. [DOI] [PubMed] [Google Scholar]
- 18.Murakami M, Kudo I, Inoue K. J Biol Chem. 1993;268:839–844. [PubMed] [Google Scholar]
- 19.Bonventre J V, Huang Z, Taheri M R, O’Leary E, Li E, Moskowitz M A, Sapirstein A. Nature (London) 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 20.Murakami M, Matsumoto R, Urade Y, Austen K F, Arm J P. J Biol Chem. 1995;270:3239–3246. doi: 10.1074/jbc.270.7.3239. [DOI] [PubMed] [Google Scholar]
- 21.Razin E, Stevens R L, Akiyama F, Schmid K, Austen K F. J Biol Chem. 1982;257:7229–7236. [PubMed] [Google Scholar]
- 22.Robinson D, Stirling J L. Biochem J. 1968;107:321. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam B K, Xu K, Atkins M B, Austen K F. Proc Natl Acad Sci USA. 1992;89:11598–11602. doi: 10.1073/pnas.89.23.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami M, Kuwata H, Amakasu Y, Shimbara S, Nakatani Y, Atsumi G, Kudo I. J Biol Chem. 1997;272:19891–19897. doi: 10.1074/jbc.272.32.19891. [DOI] [PubMed] [Google Scholar]
- 25.MacPhee M, Chepenik K P, Liddell R A, Nelson K K, Siracusa L D, Buchberg A M. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy B P, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt D E, Cromlish W A. J Biol Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 27.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Nature (London) 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 28.Roshak A, Sathe G, Marshall L A. J Biol Chem. 1994;269:25999–26005. [PubMed] [Google Scholar]
- 29.Marshall L A, Bolognese B, Winkler J D, Roshak A. J Biol Chem. 1997;272:759–765. doi: 10.1074/jbc.272.2.759. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead M V, Tischfield J A, Kudo I. J Biol Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 31.Balsinde J, Dennis E A. J Biol Chem. 1996;271:6758–6765. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- 32.Balboa M A, Balsinde J, Winstead M V, Tischfield J A, Dennis E A. J Biol Chem. 1996;271:32381–32384. doi: 10.1074/jbc.271.50.32381. [DOI] [PubMed] [Google Scholar]
- 33.Balsinde J, Balboa M A, Dennis E A. Proc Natl Acad Sci USA. 1998;95:7951–7956. doi: 10.1073/pnas.95.14.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]