Abstract
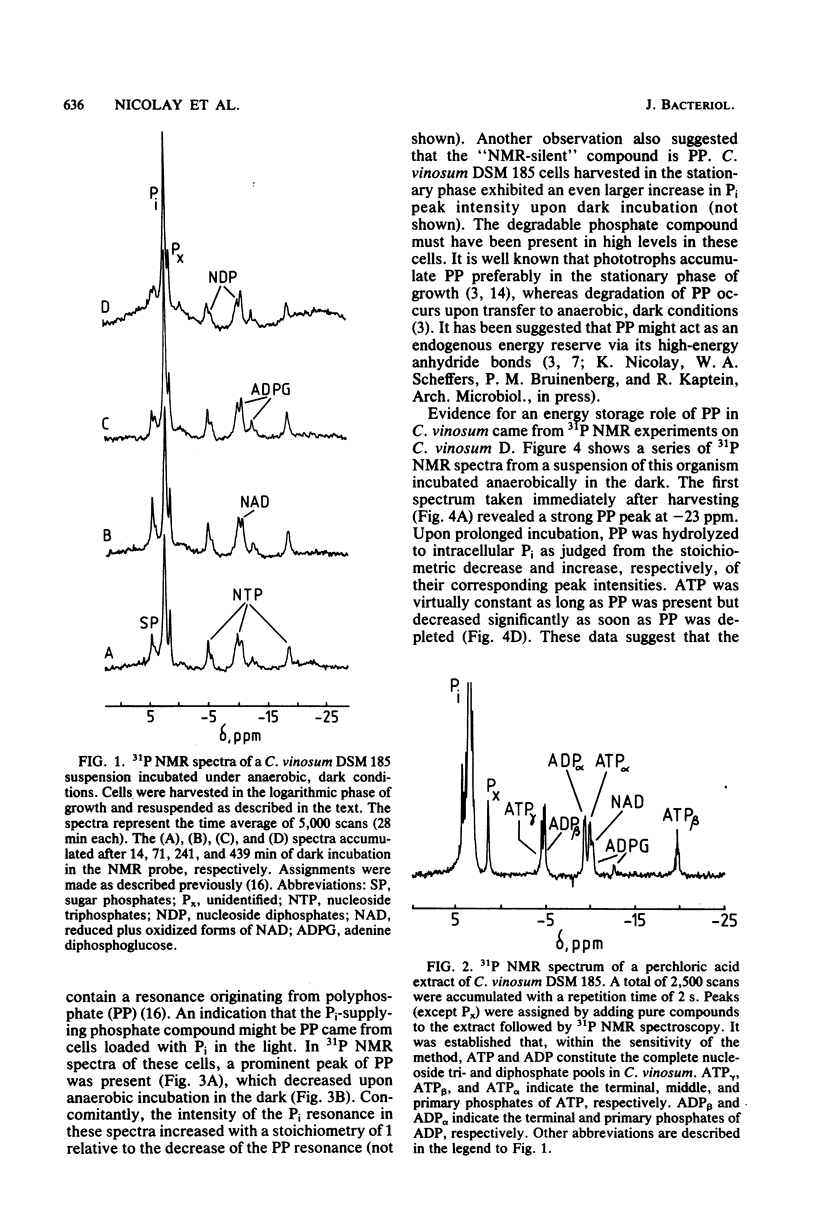
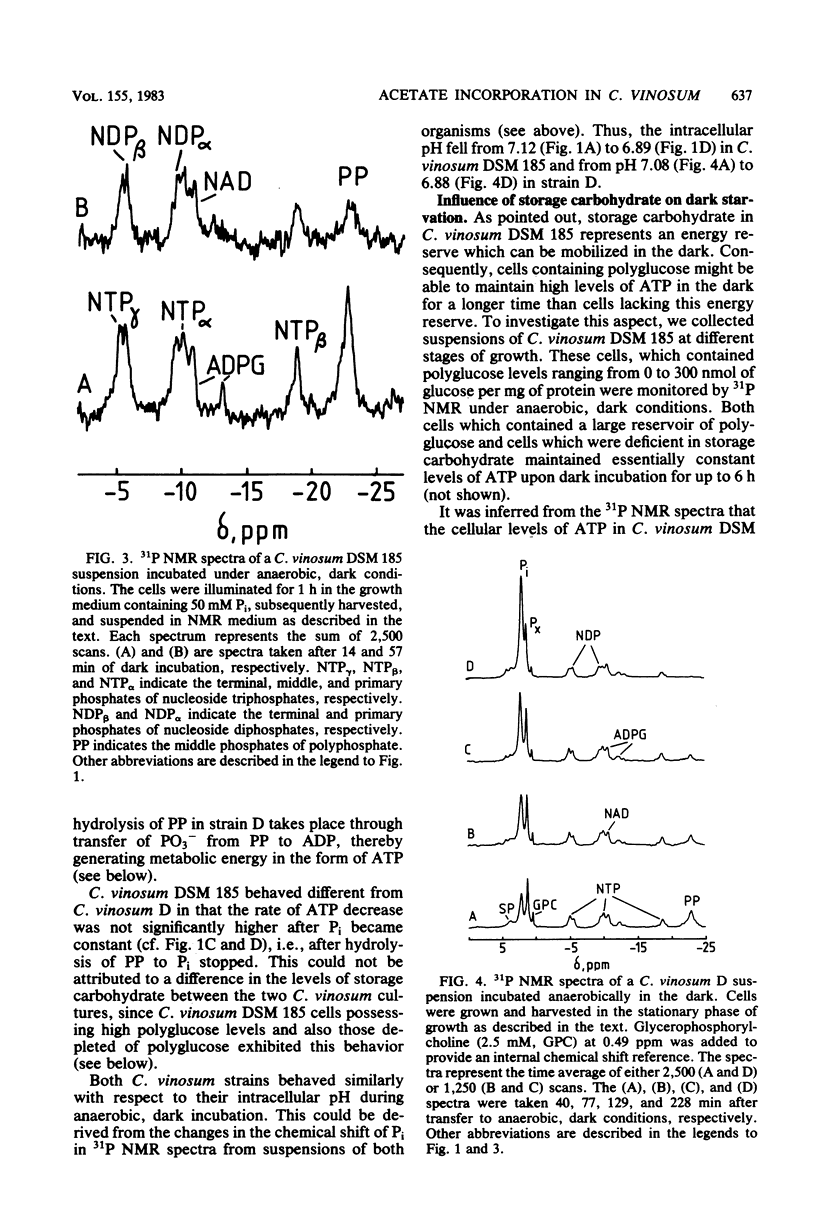
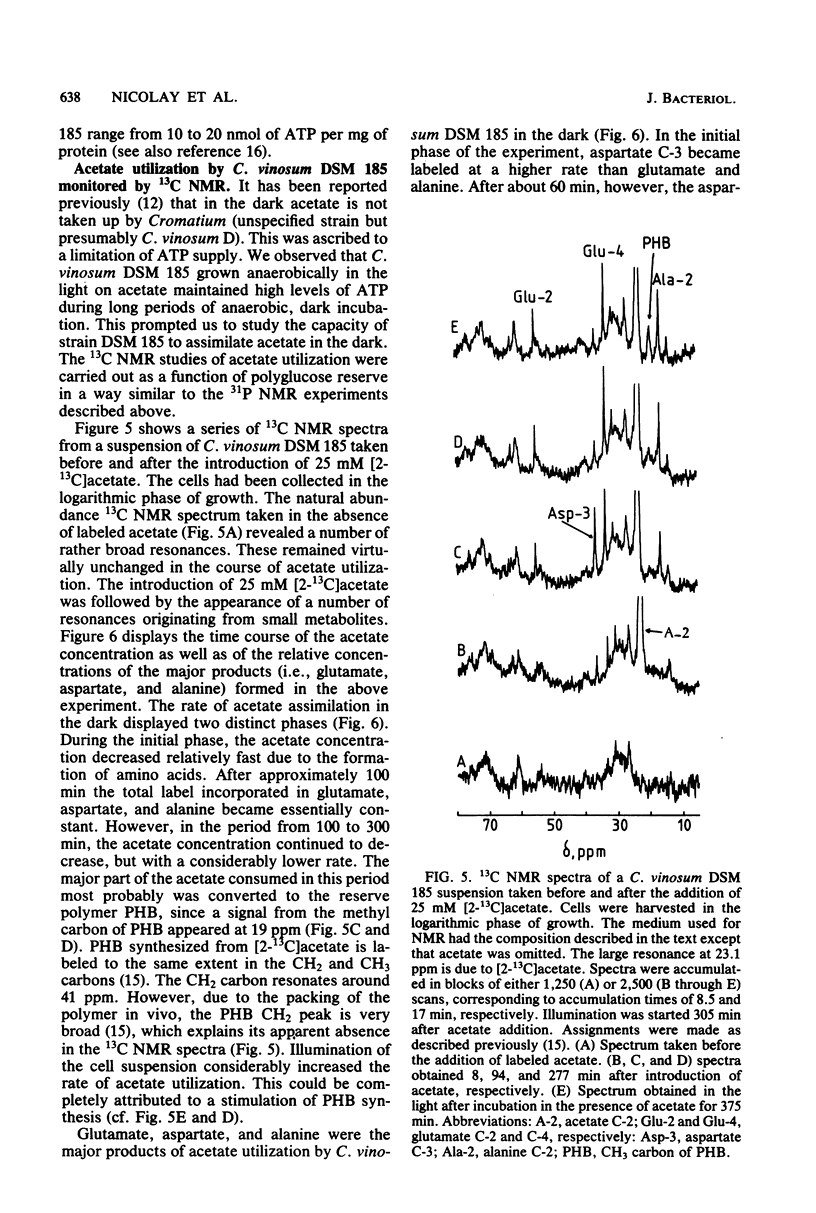
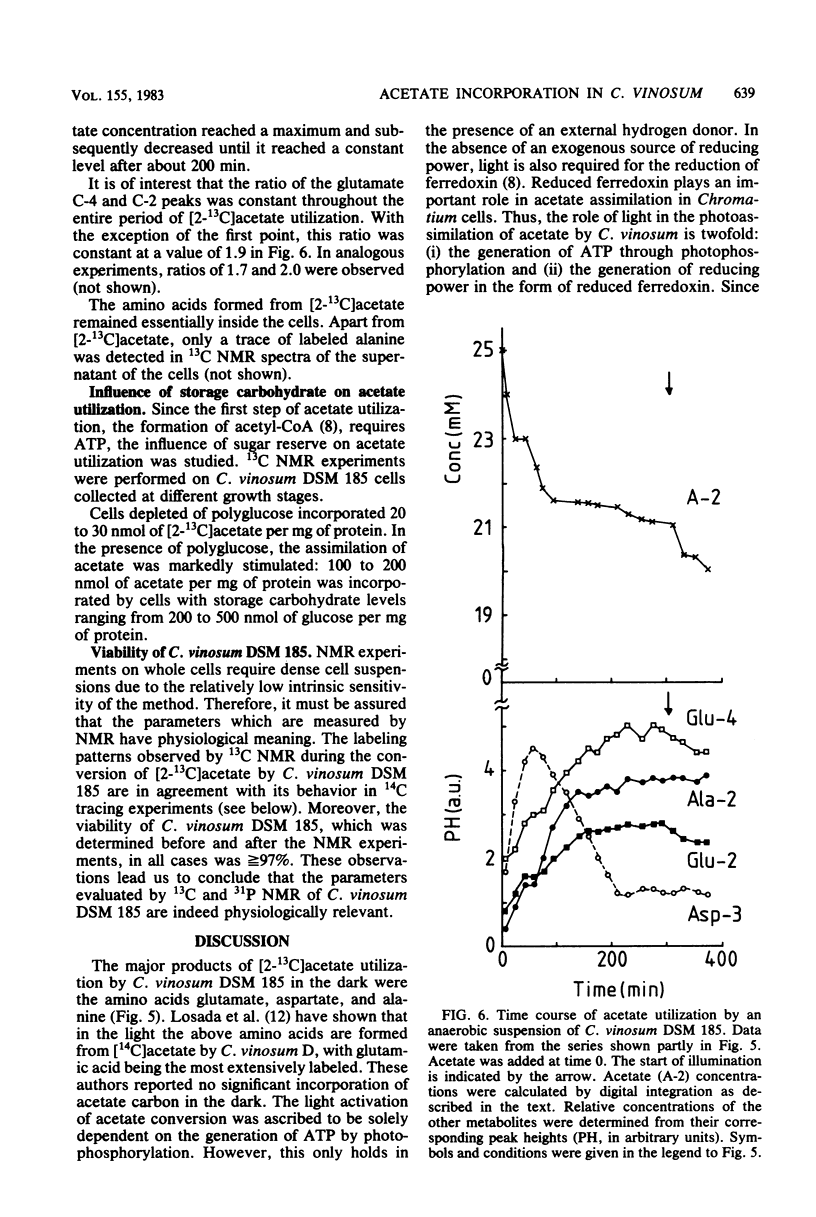
31P and 13C nuclear magnetic resonance (NMR) experiments were performed on suspensions of the phototrophic bacterium Chromatium vinosum incubated anaerobically in the dark. 31P NMR spectra revealed that during prolonged dark incubation high ATP levels are maintained. This phenomenon was independent of the presence of the energy reserves polyglucose and polyphosphate. 13C NMR experiments revealed that the amino acids glutamate, aspartate, and alanine are the major products of acetate incorporation in the dark. Apart from these amino acids, poly-beta-hydroxybutyrate was also formed. Acetate metabolism was markedly stimulated by the presence of polyglucose. The specific 13C activity of glutamate C-2 was approximately 50% that of glutamate C-4. The idea is discussed that this difference is the consequence of the maintenance of redox balance during entry of acetate into cell metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHANAN B. B., BACHOFEN R., ARNON D. I. ROLE OF FERREDOXIN IN THE REDUCTIVE ASSIMILATION OF CO2 AND ACETATE BY EXTRACTS OF THE PHOTOSYNTHETIC BACTERIUM, CHROMATIUM. Proc Natl Acad Sci U S A. 1964 Sep;52:839–847. doi: 10.1073/pnas.52.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B., Arnon D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):928–934. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B. Photoreduction of ferredoxin and its use in carbon dioxide fixation by a subcellular system from a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1420–1425. doi: 10.1073/pnas.53.6.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., SISLER E. C., KORNBERG H. L. Carbon metabolism in Chromatium. J Biol Chem. 1961 Jul;236:2140–2149. [PubMed] [Google Scholar]
- Felter S., Stahl A. J. Enzymes du métabolisme des polyphosphates dans la levure. 3. Purification et propriétés de la polyphosphate-ADP-phosphotransferase. Biochimie. 1973;55(3):245–251. doi: 10.1016/s0300-9084(73)80122-1. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Halmann M., Yariv J. The molecular composition of the volutin granule of yeast. Biochem J. 1982 Mar 1;201(3):473–479. doi: 10.1042/bj2010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOSADA M., TREBST A. V., OGATA S., ARNON D. I. Equivalence of light and adenosine triphosphate in bacterial photosynthesis. Nature. 1960 Jun 4;186:753–760. doi: 10.1038/186753a0. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Kaptein R., Hellingwerf K. J., Konings W. N. 31P nuclear magnetic resonance studies of energy transduction in Rhodopseudomonas sphaeroides. Eur J Biochem. 1981 May;116(1):191–197. doi: 10.1111/j.1432-1033.1981.tb05318.x. [DOI] [PubMed] [Google Scholar]
- van Gemerden H. On the ATP generation by Chromatium in darkness. Arch Mikrobiol. 1968;64(2):118–124. doi: 10.1007/BF00406970. [DOI] [PubMed] [Google Scholar]



