Abstract
Nuclear receptors comprise a large and expanding family of transcription factors involved in diverse aspects of animal physiology and development, the functions of which can be modulated in a spatial and temporal manner by access to small lipophilic ligands and/or the specificity of their own localized expression. Here we report the identification of a human nuclear receptor that reveals a unique proximal box (CNGCSG) in the DNA-binding domain. The conservation of this feature in its nematode counterpart suggests the requirement for this type of P box in the genetic cascades mediated by nuclear receptors in a wide variety of animal species. The expression of this receptor, PNR (photoreceptor-specific nuclear receptor), appears strongly restricted in the retina, exclusively in photoreceptor cells. In human cell lines, PNR expression was observed in Y79 retinoblastoma along with other photoreceptor marker genes such as CRX. Among vertebrate receptors, PNR shares structural kinship with an orphan receptor TLX, and despite distinct differences in the DNA binding domain, PNR is able to recognize a subset of TLX target sequences in vitro. Analyses of the human PNR gene revealed its chromosomal position as 15q24, a site that has recently been reported as a susceptible region for retinal degeneration. These data support a role for PNR in the regulation of signalling pathways intrinsic to the photoreceptor cell function.
Nuclear receptors comprise a large family of ligand-dependent transcription factors that transduce inter- and intracellular lipophilic signals to elicit genomic responses. Members of this family, as a general rule, are characterized by discrete domains that function in DNA and ligand binding. In recent years, many additional members have been identified on the basis of these structural features (the so-called orphan receptors), which in turn provided tools to search for molecules acting as specific ligands to unravel novel signaling pathways (1–3). In addition to allowing identification of the traditional steroid and thyroid hormones, retinoic acid, and vitamin D3, these efforts have culminated in the identification of a diverse group of natural and synthetic compounds as nuclear receptor ligands (ref. 2 and refs. therein). The orphan receptors can be classified into two major classes: those that heterodimerize with the retinoid X receptor and those that do not (2, 3). A notable feature of the latter class is their structural conservation during the course of metazoan evolution (4). In addition, many of these orphan receptors have been shown to be involved in regulatory gene networks that constitute fundamental genetic frameworks during embryonic development as well as in adult physiology. Some of these receptors are expressed in highly localized manner, and several instances have been shown reflecting an indispensable role for them intrinsic to these target tissues (ref. 3 and refs. therein).
We and others have shown that a unique member of this family, TLX, that is structurally and functionally homologous to the orphan receptor encoded by Drosophila terminal/gap gene tailless, shows a restricted pattern of expression in the developing fore/midbrain neuroepithelium, retina, and nasal epithelium (5, 6). Such expression of TLX in the anterior neural and sensory epithelia is apparently well conserved among vertebrates (7, 8). A recent report in Xenopus embryos indicated that inhibition of TLX function results in the interference of the evagination of the eye vesicle, demonstrating an active role for a TLX-mediated transcriptional cascade during eye development (7). On the other hand, gene knockout experiments in the mouse revealed specific defects in forebrain derivatives; however, other regions, including the eye, appear to develop normally (9). These observations suggest that the loss of TLX function in the mice might be partially compensated.
Through the search for subtypes related to TLX, we identified a nuclear receptor, PNR (photoreceptor-specific nuclear receptor), which shows localized expression in retinal photoreceptor cells. PNR is capable of binding to a subset of TLX target sequences in vitro, suggesting that they may be involved in regulation of an overlapping target-gene network. The identification of such a photoreceptor-restricted nuclear receptor implicates possible involvement of a signaling pathway regulating photoreceptor cell differentiation and/or maintenance, which may be mediated by lipophilic molecules acting as a PNR ligand.
MATERIALS AND METHODS
cDNA Cloning.
For 3′ rapid amplification of cDNA ends (RACE) reaction, cDNA was synthesized by using 1.8 pmol of T3-oligo(dT) adapter primer from 1.2 μg of poly(A)+ RNA isolated from Y79 retinoblastoma cells in total volume of 40 μl with 200 units of SuperscriptII reverse transcriptase (Life Technologies, Grand Island, NY). The reaction mixture was incubated for 30 min at 37°C, 45°C, 50°C, and 55°C successively and terminated at 70°C for 15 min. RNA was removed with 2.4 units of RNaseH for 30 min at 37°C and extracted with phenol/chloroform. Thirty cycles of PCR amplification (30 sec at 94°C, 60 sec at 60°C, 90 sec at 72°C) were carried out by using 2 μl of the synthesized cDNA, 20 pmol each of KMO4 and T3 primers and 2.5 units of Ex-Taq DNA polymerase (Takara Shuzo, Kyoto). A second round of amplification was carried out for 23 cycles using 1/50 of the first PCR mixture as template with the NMO70-T3 primer combination. The 5′ portion of the cDNA was amplified by using primers KMO19 and NMO71. These PCR products were purified and ligated into the T/A cloning vector pMOSBlue (Amersham Pharmacia). The DNA sequence was determined by ThermoSequenase sequencing kit (Amersham Pharmacia) on A.L.F. express DNA sequencer (Amersham Pharmacia). The identities of these cDNA clones were confirmed by analyzing independent RT-PCR products amplified from Y79 total RNA. Using the cDNA synthesized with NMO71 primer, 5′ RACE reactions (10) (5′ RACE system, Life Technologies, Grand Island, NY) were performed with both KMO1 and KMO20 primers and amplified DNA fragments were cloned and analyzed. T3: 5′-GCAATTAACCCTCACTAAAGGG-3′; KMO1: 5′-AGATGCCATAGTGCTTCCCGCTGC-3′; KMO4: 5′-CCATGGAGACCAGACCAACAGCTC-3′; KMO19: 5′-AGACAGAGGTTCATGGACTGAGGC-3′; KMO20: 5′-CCCATCTGCCTGGAGACTCCTTC-3′; NMO70: 5′-TCTGATGAGCTCCACAGTGGCTGC-3′; NMO71: 5′-CGTACGCTCCTCTTGAAGAAACCGC-3′.
DNA-Binding Assays.
The human PNR coding sequence was fused downstream of the hemagglutinin (HA) tag (MVYPYDVPDYA) encoded by a pCMX expression vector (11) (pCMX-HA-hPNR). COS cells were transfected with pCMX-HA-hPNR, pCMX-TLX (5), or pCMX-HA by calcium phosphate precipitation (11). Whole-cell extracts were prepared in buffer containing 20 mM Tris (pH7.5), 400 mM KCl, 2 mM DTT, 1 mM PMSF, and 20% glycerol. DNA-binding reactions were carried out in 20 μl of 10 mM Tris (pH8.0), 1 mM DTT, 0.1% Nonidet P-40, 7.5% glycerol, and 2 μg of poly(dI-dC) with COS extract (24 μg of protein). After a 30-min incubation at room temperature, 0.5–0.8 pmol of 32P-labeled double-stranded oligonucleotides was added, and incubations were continued for an additional 30 min on ice. For the supershift experiments, 2 μl each of either rabbit anti-HA antibody (sc-805) or polII antibody (sc-899) (Santa Cruz Biotechnology) was added to the reaction. For the competition experiments, unlabeled probes were added in 10- or 50-fold molar excess to the labeled probe in the binding reactions. DNA–protein complexes were resolved on a 5% polyacrylamide gel in 0.5× TBE buffer, and subsequently the gels were dried and subjected to autoradiography.
Blot Hybridization.
32P-labeled human PNR probe was prepared by randomly labeling the cDNA fragment encoding ligand-binding domain (LBD) (amino acids 248 to 410). The Zoo-blot (CLONTECH) was processed according to the manufacturer’s instructions with washing at 60°C in 0.2× standard saline phosphate/EDTA (1× = 0.18 M NaCl/10 mM phosphate, pH 7.5/1 mM EDTA), 0.1% SDS buffer. For Northern hybridization, DIG-11-dUTP (Boehringer Mannheim) was incorporated into the PNR LBD cDNA through PCR by Ex-Taq DNA polymerase (Takara Shuzo, Kyoto) with primer sets KMO25 (5′-GGATCAGGTGATCCTGCTGGAAG-3′) and KMO39 (5′-GCTTGGAAACATTTCACCTCCACCC-3′). MTN blot (CLONTECH) carrying 2 μg each of poly(A)+ RNA from various rat tissues along with 10- to 11-week-old rat retina poly(A)+ RNA (2 μg) were hybridized according to the manufacturer’s instructions with the exception that the PNR probe was washed under the same conditions used for the Southern blot. The blot was rehybridized with a β-actin probe.
In Situ Hybridization.
A 0.8-kb cDNA fragment encoding the mouse PNR (corresponds to amino acids 61–324 of the human PNR) was amplified by using reverse transcription–PCR (RT-PCR) with total RNA isolated from adult mouse eyes, gel purified, and subcloned into pGEMT-easy T/A-vector (Promega). The mouse CRX cDNA [nucleotides 928-1594 according to Furukawa et al.(12)] was subcloned into pBluescript II KS+. These plasmids were used to prepare digoxygenin-labeled antisense RNA probes. In situ hybridization of frozen tissue sections were performed as described by Ishii et al. (13).
Reverse Transcription–PCR Analysis.
Total RNA was isolated from cultured human cell lines with Isogen (Nippon Gene, Toyama, Japan), and cDNA template was prepared by using RNA PCR kit (Takara Shuzo, Kyoto) according to the manufacturer’s instructions. Using 20 pmol of each primer and 5 μl of the synthesized cDNA, PCR amplification was performed for 30 cycles (30 sec at 94°C, 60 sec at 60°C, and 90 sec at 72°C) with 2.5 units of Ex-Taq DNA polymerase (Takara Shuzo, Kyoto). The sequences of the primers are as follows; KMO4 and NMO71 for PNR, KT1 and KT2 for CRX, KMO7 and KMO8 for NRL, and NMO40 and NMO41 for retinoic acid receptor α (RARα). Products were resolved by electrophoresis in 1.5% agarose gel. KT1: 5′-GGCTCTGAAGATCAATCTGCCTGA-3′; KT2: 5′-CCATAGCTCTGGCCTGATAGGGAG-3′; KMO7: 5′-CTGCTCCATGGAGCCTTCAGTCTC-3′; KMO8: 5′-AACCGCTCTGCCAGCTGGACGTGCT-3′; NMO40: 5′-GCAGCAGTTCTGAAGAGATAGTGCC-3′; NMO41: 5′-GAGTTCACTGAACTTGTCCCAGAGG-3′.
Genomic Analysis.
A human P1 clone was obtained from Genome Systems (St. Louis) via screening with primers KMO19 and KMO20. An 8.5-kb XbaI fragment, covering all of the coding exons except exon 8, was isolated from the P1 clone and subjected to structural analysis. Exon 8 was obtained by PCR amplification from the P1 clone with primers KT9 and KMO39 and sequenced. Chromosomal mapping of the PNR genomic P1 clone was performed by using the fluorescence in situ hybridization method as described (14). The P1 clone was labeled by using the standard nick-translation with biotin-16-dUTP (Boehringer Mannheim). Signal detection and amplification were achieved by subsequent incubation with fluorescein isothiocyanate conjugated to avidin (FITC–avidin DCS). Metaphase spreads were counterstained with 1 μg/ml 4′,6-diaminido-2-phenylindole (DAPI). KT9: 5′-AGTGATGCTGAGCCAGCACAGCAA-3′.
RESULTS
A Unique Class of Nuclear Receptors.
Degenerate PCR primer sets were designed for detection of Drosophila embryonic mRNA sequences related to those encoding the TLL/TLX-type DNA-binding domain (DBD). Analyses of the PCR products identified a cDNA clone encoding a nuclear receptor DBD closely related to that of TLL, but clearly distinct (Y.U., unpublished results). We therefore searched the NCBI expressed sequence tag database (15) for the presence of a vertebrate homologue and found a highly related partial human sequence (accession no. W27871), which had been identified in an adult retina cDNA library. Based on this EST sequence, we synthesized oligonucleotide primers and screened several human cell lines by using RT-PCR. Positive signals were obtained from a retinoblastoma line, Y79, and subsequently, in combination with 3′ RACE and RT-PCR, we isolated and analyzed a full-length cDNA.
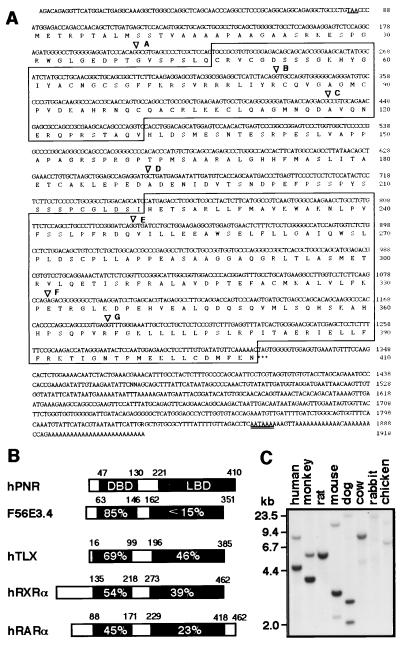
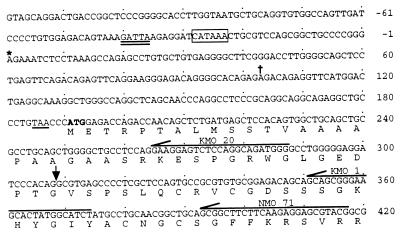
Analysis of the 1.9-kb cDNA sequence that extended to the poly(A)+ tail indicated the presence of a 1,230-bp ORF, and the deduced amino acid sequence revealed a characteristic nuclear receptor structure with a DBD followed by a putative LBD (Fig. 1A). Based on its unique expression pattern as described below, this nuclear receptor is referred to as PNR (photoreceptor-specific nuclear receptor). The calculated molecular mass of the 410-aa PNR protein is 44.7 kDa. Among known vertebrate nuclear receptors, PNR shows the highest similarity to TLX (8) both in the DBD and LBD, although the identity between their LBDs is limited (Fig. 1B). The phylogenetic conservation of PNR was examined by means of Southern hybridization by using a Zoo blot (Fig. 1C). Specific bands were readily obtainable for mammals, except rabbit, which along with chicken required a longer exposure for visualization of signals. In addition, screening of a chicken cDNA library by using the human PNR as a probe resulted in the isolation of a cDNA clone encoding a PNR-like receptor (M.K., unpublished results). During our search through the NCBI database, we found the presence of another homologous sequence in the Caenorhabditis elegans genome (F56E3.4 from GenBank U41536), indicating that the PNR DBD is well conserved during evolution. However, apparent structural similarity between PNR and F56E3.4 is absent outside the DBD.
Figure 1.
(A) The human PNR cDNA and predicted amino acid sequences. An in-frame stop codon preceding the initiation methionine codon is underlined. DBD and LBD regions are boxed. There are some sequence ambiguities in the 3′ untranslated region. A potential poly(A)+ signal (AATAAA) is double-underlined. Intron positions are indicated by ▿ (see Table 1 for details). (B) Comparison of human PNR (hPNR) with nematode F56E3.4 and other nuclear receptors. The % identity is indicated in the aligned sequences for the DBDs and LBDs. (C) Southern blot analysis using the LBD region of the human PNR cDNA as a probe. Distinct bands were dectected in all lanes.
Dimeric DNA Binding by PNR.
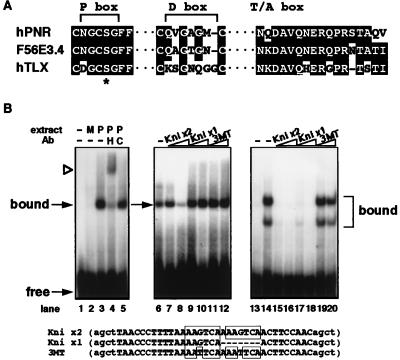
PNR shares some of the distinguishing features of the TLX DBD such as the replacement of the highly conserved lysine at the third position in the P box by a serine (5, 6) (Fig. 2A). In addition, the first amino acid in the PNR P box is asparagine rather than the usual acidic glutamic or aspartic acid residues. The PNR D box encodes 6 amino acids, compared with 7 in TLX and 5 for most others. In the T/A box or CTE (C-terminal extension) region, which has been implicated in dimerization and/or DNA sequence recognition (ref. 16 and refs. therein), PNR is closely related to TLX. These features of the PNR DBD, which are also well conserved in the C. elegans F56E3.4 protein, suggest that PNR may comprise a class of receptors that control a unique set of target genes.
Figure 2.
(A) Alignment of the amino acid residues in the P box, D box and T/A box regions of the DBDs between human PNR, C. elegans F56E3.4 and human TLX. Conserved residues are shaded. Gaps are indicated by a dashed line. ∗ indicates the position of the serine residue in the P boxes of these 3 receptors that replaces an otherwise strictly conserved lysine. (B) In vitro DNA binding specificity by PNR protein. Lanes 1–5 show DNA-binding patterns of COS cell-expressed HA-tagged PNR protein to Kni x2 probe. Lane 1 is probe only, and mock cell extract was added in lane 2. Lane 3 shows a specific protein–DNA complex formed when cell extract containing HA-hPNR is added. This band is supershifted by addition of antibody against the HA tag (lane 4) but is not affected by nonspecific antibody (lane 5). Lanes 6–12 show that PNR bound to labeled Kni x2 probe can be blocked by competition by addition of excess Kni x2 cold probes (lane 8), but not by Kni x1, which is based on the TLL binding site identified in the D. melanogaster knirps gene (17), or 3MT probes (lanes 9–12). Competitor probes are indicated above, with 10-fold excess added in lanes 7, 9, and 11 and 50-fold excess in lanes 8, 10, and 12. Lanes 13–20 show that COS cell-expressed chicken TLX protein forms two distinct complexes on the Kni x2 probe (lane 14), both of which can be efficiently blocked by competition by either Kni x2 or Kni x1, but not the mutated 3MT probe.
To begin to address this, we examined whether PNR had the ability to recognize the TLL/TLX binding site, albeit the differences in the P and D boxes. Gel-shift analysis using COS cell-expressed HA-tagged PNR protein indicated that it is unable to bind the TLL/TLX target sequence that encodes a single copy of an AAGTCA half-site [Kni x1, based on a TLL binding site identified in the upstream region of Drosophila melanogaster knirps gene (17)] (Fig. 2B). This brought up the following possibilities; PNR may recognize a completely distinct half-site sequence, or it may require the presence of more than one half-site to form a stable complex. Substitution of the nucleotides within the half-site sequence did not result in binding by PNR (data not shown), however, when we tested a dimeric direct repeat containing two AAGTCA half-sites separated by one spacer nucleotide (Kni x2), a distinct protein–DNA complex was readily detectable. This complex could be supershifted with HA-antibody, confirming the presence of PNR in the complex. Competition experiments further revealed that the binding of PNR to this direct repeat sequence is specific, because oligonucleotides encoding either the single half-site (Kni x1) or mutated half-sites (AATTCA, 3MT) failed to compete, suggesting that PNR forms dimers (Fig. 2B). By using the Kni x2 probe, COS cell-expressed TLX formed two distinct protein–DNA complexes (Fig. 2B), which apparently correspond to monomers (Lower) and dimers (Upper). These two TLX complexes could be blocked by competition by either Kni x2 or Kni x1, but not by 3MT oligonucleotides, supporting the idea that TLX recognizes a single-copy half-site. This result is consistent with the idea that the mobility of the PNR–DNA complex more closely resembles that of the TLX dimer complex, further supporting the idea that PNR requires dimerization to bind the TLX-type half-site sequence. These results also indicated that the substitution of the first amino acid in the PNR P box, aspartic acid to asparagine, seems permissive with regard to half-site recognition.
PNR Expression Is Photoreceptor-Specific.
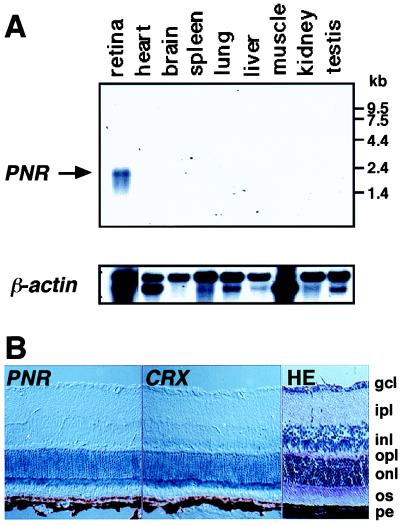
To obtain insight into the function of PNR, we examined its pattern of expression. Northern blot analysis was performed against rodent tissue RNAs using the human PNR cDNA as a probe (Fig. 3A). The tissue distribution of PNR mRNA revealed a remarkable restriction to the retina. This is consistent with the fact that the original expressed sequence tag clone was identified from a retinal cDNA library. Other tissues, including brain, were negative even after long exposure of the blot. Northern analysis against RNA from Y79 retinoblastoma cells, from which PNR cDNA was isolated, revealed the presence of a 2.0-kb transcript (data not shown), a size that correlates well with that seen in the rodent retinal RNA preparation.
Figure 3.
Expression of PNR mRNA in rodents. (A) Northern hybridization analyses of mRNA from rat tissues using the human PNR cDNA as a probe. All lanes contain 2 μg of poly(A)+ RNA. β-Actin was used to normalize for RNA quality. (B) In situ hybridization of mouse retina sections with digoxigenin-labeled PNR and CRX RNA probes. An adjacent section was stained with hematoxylin/eosin (HE) to help visualize different cell layers. gcl, ganglion cell layer; ipl, inner plexiform layer; inl, inner nuclear layer; opl, outer plexiform layer; onl, outer nuclear layer; os, outer segments; pe, pigment epithelium.
Because the retina consists of several distinct cell layers, in situ hybridization was used to observe a more detailed pattern of localization. For this pupose, we obtained a partial mouse PNR cDNA fragment by using RT-PCR from adult mouse eye RNA. Examination of eye sections from 2-week-old mice revealed that PNR expression is clearly localized to the outer nuclear layer of the retina, which contains the nuclei of cone and rod photoreceptor cells (Fig. 3B). No signal was observed in any other layer. This staining pattern resembles that of CRX, which has been shown to be a photoreceptor-specific transcription factor (12, 18, 19).
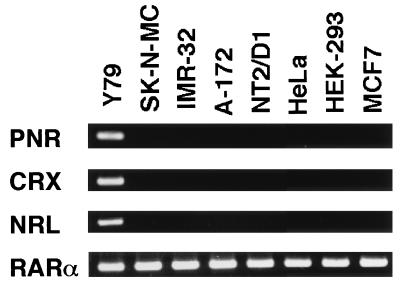
To examine the possibility of linkage between the expression of PNR and CRX, human cell lines were scanned for the presence of transcripts for both genes along with NRL, which has also been reported to be expressed in photoreceptor cells (20). Reverse transcription–PCR using specific primer sets revealed that all three genes are coexpressed in Y79 retinoblastoma cells but absent in other cell lines tested, suggesting that PNR may constitute a part of the photoreceptor-specific transcription factor cascade (Fig. 4).
Figure 4.
Expression of PNR, CRX, NRL, and RARα in human cell lines. RT-PCR analysis of total RNA isolated from various human cell lines. Y79, retinoblastoma; SK-N-MC and IMR-32, neuroblastoma; A-172, glioblastoma; NT2/D1, embryonic carcinoma; HeLa, cervical carcinoma; HEK-293, adenovirus-transformed embryonic kidney fibroblast; and MCF7, mammary tumor.
Structure of the Human PNR Gene.
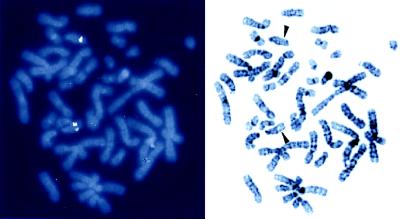
The restricted expression of PNR indicated that this gene likely encodes regulatory elements which direct photoreceptor-specific transcription and that its locus might be involved in heritable human diseases affecting vision. To address these issues, a bacterial P1 phage clone encoding the human PNR locus was obtained, and the transcription unit and chromosomal localization were analyzed. The PNR-coding region is divided into 8 exons spanning 7 kb within the approximately 65-kb P1 insert (Table 1). The position of PNR introns A, C, and E appears to be homologous to those in the TLX gene (8). The deduced PNR-coding sequences were identical to those obtained from the Y79 transcripts by using RT-PCR. To determine the 5′ end of the transcripts, 5′ RACE was performed on Y79 RNA. The longest clones extended 190 bp upstream of the methionine codon (Fig. 5), which predicts the size of the mature mRNA to be about 2 kb, consistent with our Northern data. Inspection of sequences in this vicinity revealed the presence of a TATA box-like sequence, and preliminary analysis indicated that a 2.2-kb fragment encompassing this region has promoter activity in Y79 cells (data not shown). By using the P1 clone, fluorescence in situ hybridization analyses were performed to determine the chromosomal location of the PNR gene. In all of the normal metaphase chromosomal preparations tested, specific signals were obtained, identifying the locus as 15q24 (Fig. 6). Among known genetic loci of which polymorphisms have been linked to congenital retinal disorders, 15q24 was recently identified as a susceptible region for a heritable retinal degeneration associated with mental retardation and spasticity (21).
Table 1.
Sequence at exon/intron junctions
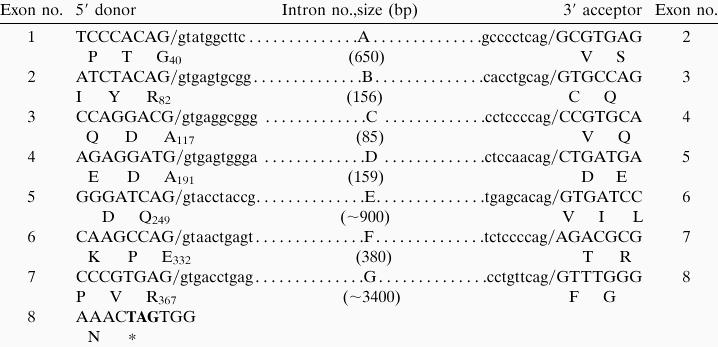 |
Figure 5.
Primary structure of the 5′ upstream region of the human PNR gene. The end of the longest 5′ RACE clones is indicated by ∗ (denoted as +1 in the numbering of the nucleotides) and the upstream TATA box (CATAAA) is shown (boxed). The 5′ end of the cDNA in Fig. 1A is indicated (†). The first ATG is in bold, with an in-frame upstream stop codon underlined. The junction between the first and second exons is shown by the arrow, and the positions of the primers used in the 5′ RACE analysis are marked and labeled. A CRX consensus binding sequence just upstream of the TATA box is indicated by double-underline.
Figure 6.
Chromosomal localization of the human PNR gene. (Left) A representative fluorescence in situ hybridization pattern obtained with the human PNR P1 genomic clone as a probe. (Right) The same metaphase chromosome preparation stained with 4′,6-diaminido-2-phenylindole (DAPI). The human PNR gene was assigned to 15q24 (arrowheads).
DISCUSSION
The overall structure of the newly identified PNR is most closely related to TLX among known vertebrate nuclear receptors. In this respect, PNR can be classified into the receptor subfamily II consisting of TLX, RXR, COUP, HNF-4, and TR2, most of which are known to form homodimers on binding to DNA (4). In addition to the presence of homologous orphan receptors in D. melanogaster (22), the availability of the recently completed C. elegans genome sequences (23) allowed us to examine phylogenetic conservation of these receptors. Database search revealed that in addition to F56E3.4 for PNR, a distinct ortholog also exists for TLX (C08F8.8 or NHR-67, from EMBL Z73103), indicating that both receptors are under evolutionary constraint. In both cases, the structural conservation is limited within the DBDs, and extreme diversity in the C-terminal portions leave it questionable whether these regions encode structural motifs sufficient to compose LBDs. This absence of characteristic LBD structure seems rather common among nematode nuclear receptors (24). Together, these observations support the idea that PNR and TLX each represents a unique branch through diversification of the nuclear receptor family.
PNR, as a vertebrate receptor that encodes an asparagine at the first position of the P box, suggests that further complexity can be imparted in the target gene specificity of the nuclear receptors. Nevertheless, PNR shares a TLX peculiarity, the substitution of the otherwise universally conserved lysine residue by a serine, which in TLX, seems to allow preferential recognition of the AAGTCA site (5, 17, 25). This combination of features presented us with the opportunity to examine the effect of individual P box residues. Our results demonstrated that PNR is indeed capable of binding to a DNA fragment encoding the AAGTCA site. In this regard, the choice of asparagine or aspartic acid at the first position of the P box seems permissive for overlapping binding specificity, which is in agreement with results obtained from detailed point mutagenesis studies of the P box sequences (26, 27). Nonetheless, conservation of the asparagine in the P box of PNR and F56E3.4 suggests a unique mode of half-site recognition, and identification of Kni x2 as a PNR-binding site should help in further analysis of this issue. As PNR appears to require dimeric half-sites and the apparent mobility of the PNR–DNA complex compared with that of TLX on the gel indicated that PNR binds to this probe as a dimer. We believe that PNR forms homodimers because PNR protein, prepared through reticulocyte lysate translation in vitro, also gave rise to the complex indistinguishable from that obtained with the COS cell extract (data not shown). This fits with the observation that most members of the receptor subfamily II tend to form stable homodimers and distinguishes PNR from TLX in the mode of target gene recognition. It would thus appear that PNR target genes comprise a subset, if not all, of the TLX targets, suggesting possible redundancy for PNR and TLX in cells in which they are coexpressed.
The most remarkable feature of PNR is its highly restricted expression within the eye, in the outer nuclear layer of retinal photoreceptor cells. In addition to those tissues shown in Fig. 3, we have examined detailed RNA blots (human MTN blots, CLONTECH) of the human central nervous system, cardiopulmonary, gastrointestinal, reproductive, endocrine, immune, hematopoietic, and connective tissues, which were all negative for PNR expression (data not shown). Although we were unable to obtain human retinal samples, the original (W27871) and one additional (W27698 in NCBI database, matching to the 3′ end portion of the PNR cDNA sequences) expressed sequence tag clones were both identified from retinal cDNA libraries, confirming PNR expression in the human retina. In this regard, it is of interest to see whether PNR is expressed in pinealocytes. Among transcription factors, such restricted expression has been reported only for CRX (12, 18, 19) and ERX (28), both of which encode homeodomains belonging to the orthodenticle/Otx and empty spiracle/Emx class, respectively. It has been shown for CRX, which in humans represents a responsible gene for a cone rod dystrophy classified as CORDII (19, 29), that ectopic expression in mouse retina leads to stimulation of photoreceptor cell differentiation, and a dominant-negative form can block this process (12). These observations point to an indispensable role of CRX in controlling the photoreceptor cell fate. We have found that PNR is coexpressed together with CRX both in the mouse retina ONL and Y79 retinoblastoma cells. Furthermore, our preliminary observations indicate that during embryonic retinal development in the mouse, the onset of CRX expression precedes that of PNR (data not shown). It is interesting to note that upstream of the presumed TATA box in the human PNR promoter, a potential CRX binding site is present (Fig. 5). Together, these results suggest possible regulation of the photoreceptor-specific expression of PNR by CRX.
Transgenic mouse experiments have identified a discrete set of enhancers within the opsin, interphotoreceptor retinoid-binding protein, and arrestin genes capable of directing photoreceptor-specific expression, to which CRX and ERX can bind and transactivate in vitro (12, 18, 19). Another important cis-element in the rhodopsin promoters can be recognized by NRL, a retina-enriched bZIP transcription factor, which can either transactivate by itself or in synergy with CRX (18, 30, 31). In Y79 cells, CRX, ERX, NRL, and PNR are all coexpressed (20, 28, 30, and this work), suggesting that PNR is an essential part of the photoreceptor cell-specific transcription cascade. However, inspection of these cis-elements did not reveal the presence of potential PNR-binding sequences. Then how might PNR be involved in this genetic cascade? In contrast to those transactivators, the PNR LBD confers repression when expressed in fibroblasts as a fusion to GAL4 DBD (data not shown). If this reflects the situation in the retina, the function of PNR may be to suppress genes of which expression is refractory to photoreceptor function.
The fact that PNR represents a unique nuclear receptor adds a new dimension to the complexity underlying photoreceptor cell development and/or maintenance, implicating involvement of as-yet-unidentified lipophilic molecules that may act as a ligand for PNR. It should be kept in mind that photoreceptors and the flanking retinal pigment epithelial cells support a highly active and unique cascade for the metabolism and transport of retinoids, which are essential to maintenance of retinal function (32); thus, these cells should contain a rich source of potential nuclear receptor ligands. Identification of the photoreceptor-specific PNR offers a potential tool to investigate novel signaling pathways. In addition, further analysis within the chromosomal region 15q24 will help to clarify whether the PNR locus is involved in the genetic polymorphisms associating with congenital pigmentary retinopathy.
Acknowledgments
We thank Drs. T. Furukawa and C. L. Cepko for the generous gift of the mouse CRX cDNA clone. This work was supported in part by Research for the Future from Japan Society for the Promotion of Science (Japan) and Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture (Japan).
ABBREVIATIONS
- DBD
DNA binding domain
- LBD
ligand binding domain
- RACE
rapid amplification of cDNA ends
- PNR
photoreceptor-specific nuclear receptor
- RT-PCR
reverse transcription–PCR
Footnotes
References
- 1.Enmark E, Gustafsson J A. Mol Endocrinol. 1996;10:1293–1307. doi: 10.1210/mend.10.11.8923456. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg B, Evans R M. Genes Dev. 1998;12:3149–3155. doi: 10.1101/gad.12.20.3149. [DOI] [PubMed] [Google Scholar]
- 3.Willy P J, Mangelsdorf D J. Horm Signal. 1998;1:307–358. [Google Scholar]
- 4.Escriva H, Safi R, Hanni C, Langlois M C, Saumitou-Laprade P, Stehelin D, Capron A, Pierce R, Laudet V. Proc Natl Acad Sci USA. 1997;94:6803–6808. doi: 10.1073/pnas.94.13.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu R T, McKeown M, Evans R M, Umesono K. Nature (London) 1994;370:375–379. doi: 10.1038/370375a0. [DOI] [PubMed] [Google Scholar]
- 6.Monaghan A P, Grau E, Bock D, Schütz G. Development (Cambridge, UK) 1995;121:839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 7.Hollemann T, Bellefroid E, Pieler T. Development (Cambridge, UK) 1998;125:2425–2432. doi: 10.1242/dev.125.13.2425. [DOI] [PubMed] [Google Scholar]
- 8.Jackson A, Panayiotidis P, Foroni L. Genomics. 1998;50:34–43. doi: 10.1006/geno.1998.5270. [DOI] [PubMed] [Google Scholar]
- 9.Monaghan A P, Bock D, Gass P, Schwäger A, Wolfer D P, Lipp H-P, Schütz G. Nature (London) 1997;390:515–517. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- 10.Frohman M A, Dush M K, Martin G R. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umesono K, Murakami K K, Thompson C C, Evans R M. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa T, Morrow E M, Cepko C L. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 13.Ishii Y, Fukuda K, Saiga H, Matsushita S, Yasugi S. Dev Growth Differ. 1997;39:643–653. doi: 10.1046/j.1440-169x.1997.t01-4-00012.x. [DOI] [PubMed] [Google Scholar]
- 14.Taniwaki M, Nishida K, Ueda Y, Misawa S, Nagai M, Tagawa S, Yamagami T, Sugiyama H, Abe M, Fukuhara S, Kashima K. Blood. 1995;85:3223–3228. [PubMed] [Google Scholar]
- 15.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Khorasanizadeh S, Miyoshi Y, Lazar M A, Rastinejad F. Mol Cell. 1998;1:849–861. doi: 10.1016/s1097-2765(00)80084-2. [DOI] [PubMed] [Google Scholar]
- 17.Pankratz M J, Busch M, Hoch M, Seifert E, Jäckle H. Science. 1992;255:986–989. doi: 10.1126/science.1546296. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Wang Q L, Nie Z, Sun H, Lennon G, Copeland N G, Gilbert D J, Jenkins N A, Zack D J. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 19.Freund C L, Gregory-Evans C Y, Furukawa T, Papaioannou M, Looser J, Ploder L, Bellingham J, Ng D, Herbrick J A, Duncan A, et al. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 20.Swaroop A, Xu J, Pawar H, Jackson A, Skolnick C, Agarwal N. Proc Natl Acad Sci USA. 1992;89:266–270. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell S J, McHale D P, Campbell D A, Lench N J, Mueller R F, Bundey S E, Markham A F. Am J Hum Genet. 1998;62:1070–1076. doi: 10.1086/301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thummel C S. Cell. 1995;83:871–877. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 23.Aincough R, Bardill S, Barlow K, Basham V, Baynes C, Beard L, Beasley A, Berks M, Bonfield J, Brown J, et al. Science. 1998;282:2012–2018. [Google Scholar]
- 24.Clarke N D, Berg J M. Science. 1998;282:2018–2022. doi: 10.1126/science.282.5396.2018. [DOI] [PubMed] [Google Scholar]
- 25.Hoch M, Gerwin N, Taubert H, Jäckle H. Science. 1992;256:94–97. doi: 10.1126/science.1348871. [DOI] [PubMed] [Google Scholar]
- 26.Zilliacus J, Carlstedt-Duke J, Gustafsson J A, Wright A P. Proc Natl Acad Sci USA. 1994;91:4175–4179. doi: 10.1073/pnas.91.10.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson C C, Hendy S C, Romaniuk P J. J Biol Chem. 1995;270:16981–16987. doi: 10.1074/jbc.270.28.16981. [DOI] [PubMed] [Google Scholar]
- 28.Martinez J A, Barnstable C J. Biochem Biophys Res Comm. 1998;250:175–180. doi: 10.1006/bbrc.1998.9261. [DOI] [PubMed] [Google Scholar]
- 29.Swain P K, Chen S, Wang Q L, Affatigato L M, Coats C L, Brady K D, Fishman G A, Jacobson S G, Swaroop A, Stone E, et al. Neuron. 1997;19:1329–1336. doi: 10.1016/s0896-6273(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 30.Rehemtulla A, Warwar R, Kumar R, Ji X, Zack D J, Swaroop A. Proc Natl Acad Sci USA. 1996;93:191–195. doi: 10.1073/pnas.93.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R, Chen S, Scheurer D, Wang Q-L, Duh E, Sung C H, Rehemtulla A, Swaroop A, Adler R, Zack D J. J Biol Chem. 1996;271:29612–29618. doi: 10.1074/jbc.271.47.29612. [DOI] [PubMed] [Google Scholar]
- 32.Wright A F. Nat Genet. 1997;17:132–134. doi: 10.1038/ng1097-132. [DOI] [PubMed] [Google Scholar]