Abstract
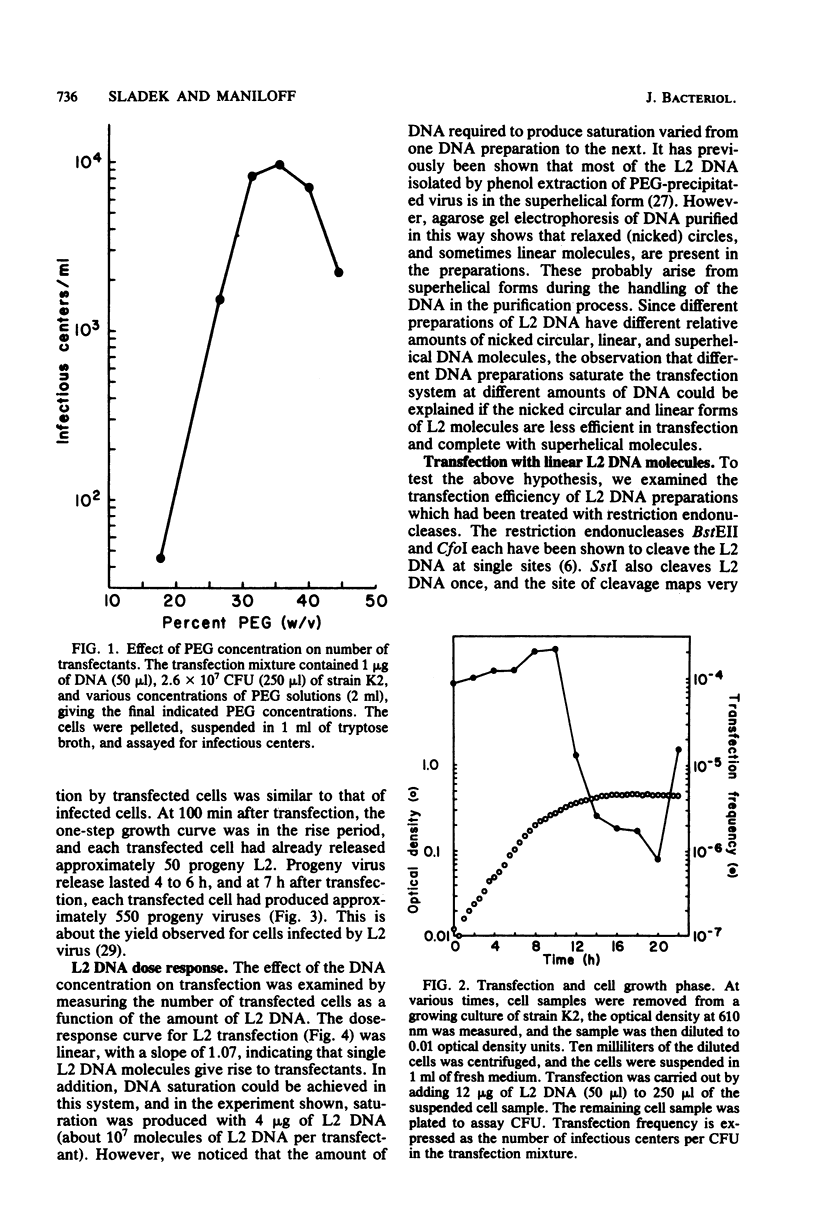
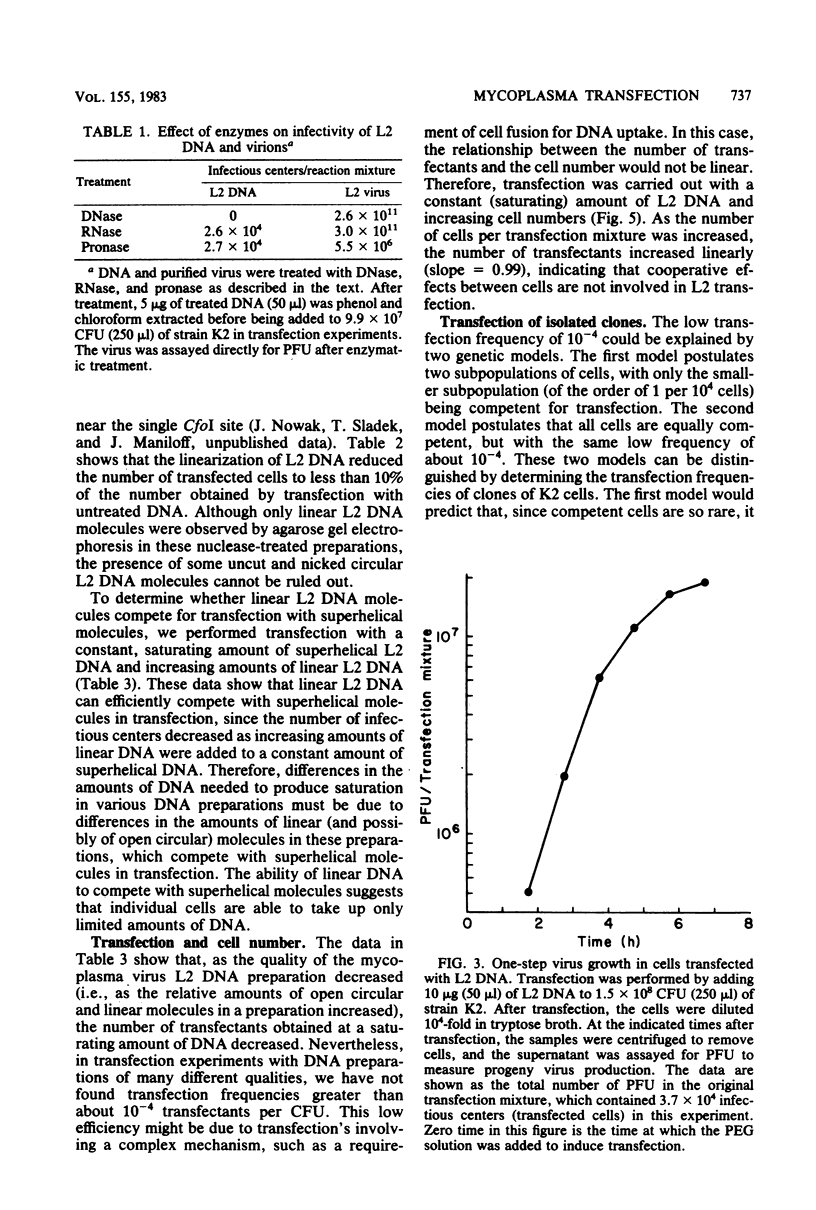
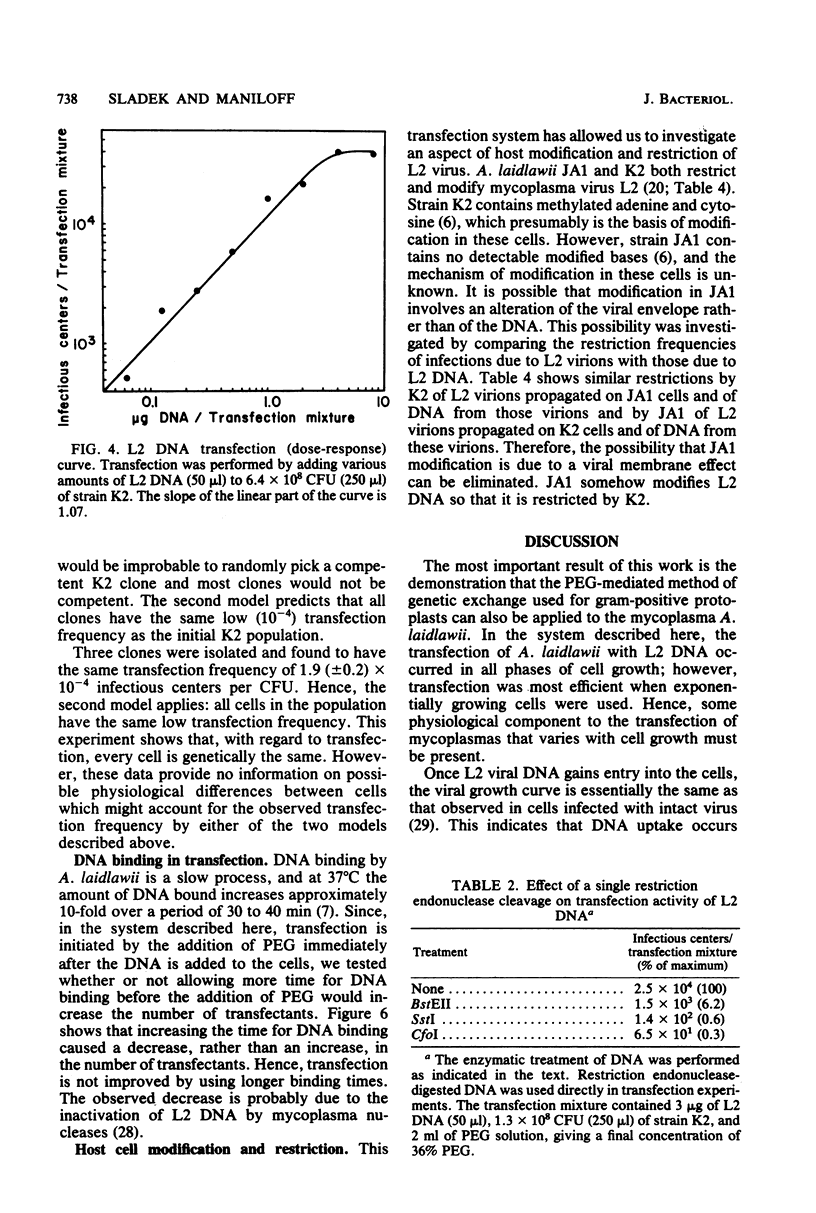
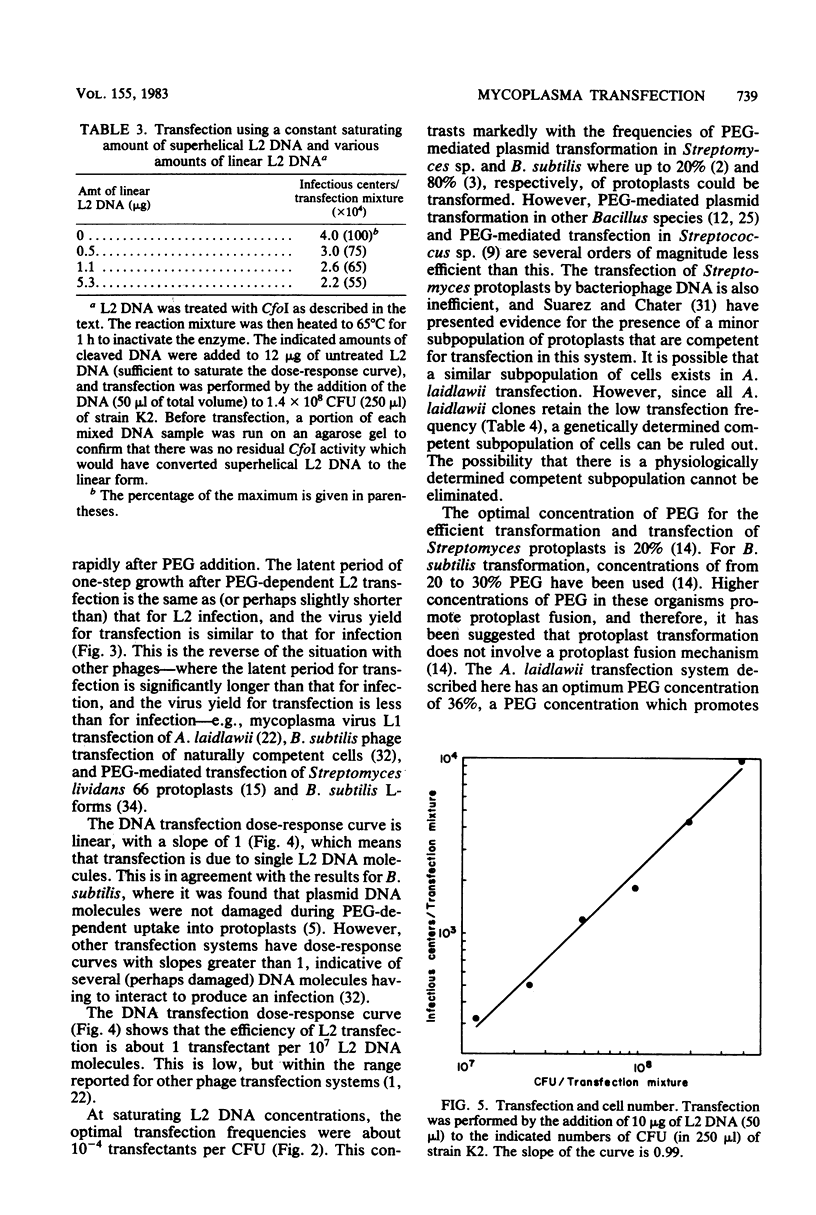
Phenol-extracted DNA from mycoplasma virus L2 was able to transfect Acholeplasma laidlawii in the presence of polyethylene glycol. Transfection was sensitive to DNase and was most efficient with 36% (wt/vol) polyethylene glycol 8000 and cells in logarithmic growth. Virus production by the transfected cells was similar to that of the cells infected by intact virus. L2 DNA transfected A. laidlawii with a single-hit dose-response curve, reaching saturation at high DNA concentrations. Optimum transfection frequencies were about 10(-7) transfectants per L2 DNA molecule and 10(-4) transfectants per CFU. When DNA was present in saturating amounts, the number of transfectants increased linearly with the number of CFU present in the transfection mixture, suggesting that DNA uptake does not occur by a mechanism involving cell fusion. The cleavage of the superhelical mycoplasma virus L2 genome with restriction endonucleases that cleave the DNA molecule once reduced the transfection frequency. Host cell modification and restriction of transfecting L2 DNA were similar to those for infecting L2 virions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzinger R. Transfection of Enterobacteriaceae and its applications. Microbiol Rev. 1978 Mar;42(1):194–236. doi: 10.1128/mr.42.1.194-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Swinton D., Maniloff J., Hattman S. Cytosine methylation of the sequence GATC in a mycoplasma. J Bacteriol. 1982 Sep;151(3):1420–1424. doi: 10.1128/jb.151.3.1420-1424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsome C. E. Deoxyribonucleate binding and transformation in Mycoplasma laidlawii. J Gen Microbiol. 1968 Jan;50(1):43–53. doi: 10.1099/00221287-50-1-43. [DOI] [PubMed] [Google Scholar]
- Furness G., Cerone A. M. Preparation of competent single-cell suspensions of Mycoplasma hominis tets and Mycoplasma salivarium tets for genetic transformation to tetracycline resistance by DNA extracted from Mycoplamsa hominis tetr. J Infect Dis. 1979 Apr;139(4):444–451. doi: 10.1093/infdis/139.4.444. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Mycoplasmatales virus-laidlawii 2, a new virus isolated from Acholeplasma laidlawii. J Gen Virol. 1971 Jul;12(1):65–67. doi: 10.1099/0022-1317-12-1-65. [DOI] [PubMed] [Google Scholar]
- Greenberg N., Rottem S. Composition and molecular organization of lipids and proteins in the envelope of mycoplasmavirus MVL2. J Virol. 1979 Dec;32(3):717–726. doi: 10.1128/jvi.32.3.717-726.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic studies with bacterial protoplasts. Annu Rev Microbiol. 1981;35:237–272. doi: 10.1146/annurev.mi.35.100181.001321. [DOI] [PubMed] [Google Scholar]
- Krügel H., Fiedler G., Noack D. Transfection of protoplasts from Streptomyces lividans 66 with actinophage SH10 DNA. Mol Gen Genet. 1980 Jan;177(2):297–300. doi: 10.1007/BF00267442. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Transfection mediated by Mycoplasmatales viral DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3423–3427. doi: 10.1073/pnas.69.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi-Meyrueis C., Fodor K., Schaeffer P. Polyethyleneglycol-induced transformation of Bacillus subtilis protoplasts by bacterial chromosomal DNA. Mol Gen Genet. 1980;179(3):589–594. doi: 10.1007/BF00271749. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Haberer K., Gourlay R. N., Das J., Cole R. Mycoplasma viruses. Intervirology. 1982;18(4):177–188. doi: 10.1159/000149323. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Lohr J. R., Dean D. H. Transformation of Bacillus thuringiensis protoplasts by plasmid deoxyribonucleic acid. J Bacteriol. 1981 Feb;145(2):980–983. doi: 10.1128/jb.145.2.980-983.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J. A., Das J., Maniloff J. Characterization of an Acholeplasma laidlawii variant with a REP- phenotype. J Bacteriol. 1976 Aug;127(2):832–836. doi: 10.1128/jb.127.2.832-836.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J. A., Maniloff J. Physical characterization of the superhelical DNA genome of an enveloped mycoplasmavirus. J Virol. 1979 Jan;29(1):374–380. doi: 10.1128/jvi.29.1.374-380.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzrath R. M., Maniloff J. Growth of an enveloped mycoplasmavirus and establishment of a carrier state. J Virol. 1977 May;22(2):308–314. doi: 10.1128/jvi.22.2.308-314.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. Polyethylene glycol-assisted transfection of Streptomyces protoplasts. J Bacteriol. 1980 Apr;142(1):8–14. doi: 10.1128/jb.142.1.8-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Spatz H. C. Transfection in B. subtilis. Curr Top Microbiol Immunol. 1973;62:61–88. doi: 10.1007/978-3-642-65772-6_3. [DOI] [PubMed] [Google Scholar]
- White T. B., Doyle R. J., Streips U. N. Transformation of a Bacillus subtilis L-form with bacteriophage deoxyribonucleic acid. J Bacteriol. 1981 Feb;145(2):878–883. doi: 10.1128/jb.145.2.878-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Andersen B. J., Sutherland B. M. Ability of Bacillus subtilis protoplasts to repair irradiated bacteriophage deoxyribonucleic acid via acquired and natural enzymatic systems. J Bacteriol. 1981 Sep;147(3):949–953. doi: 10.1128/jb.147.3.949-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos W. M., Venema G. Fate of plasmid DNA in transformation of Bacillus subtilis protoplasts. Mol Gen Genet. 1981;182(1):39–43. doi: 10.1007/BF00422764. [DOI] [PubMed] [Google Scholar]



