Abstract
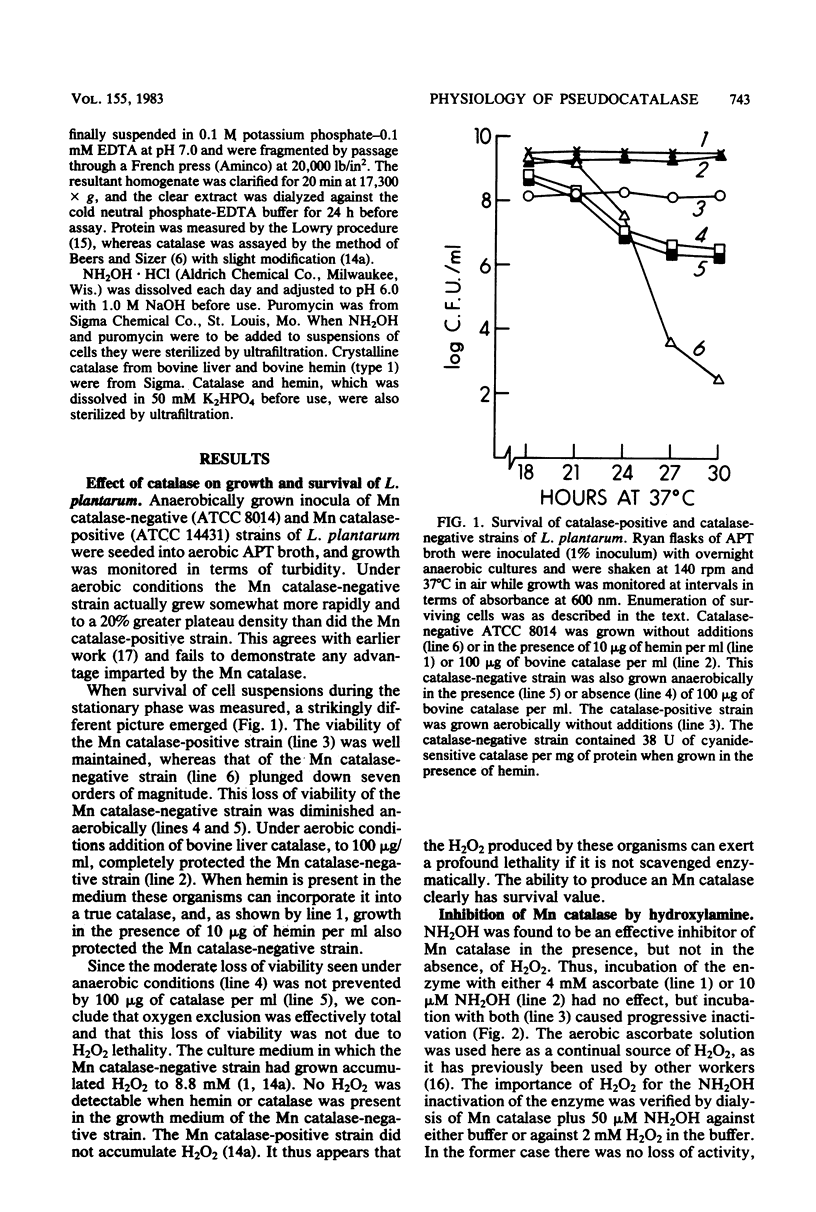
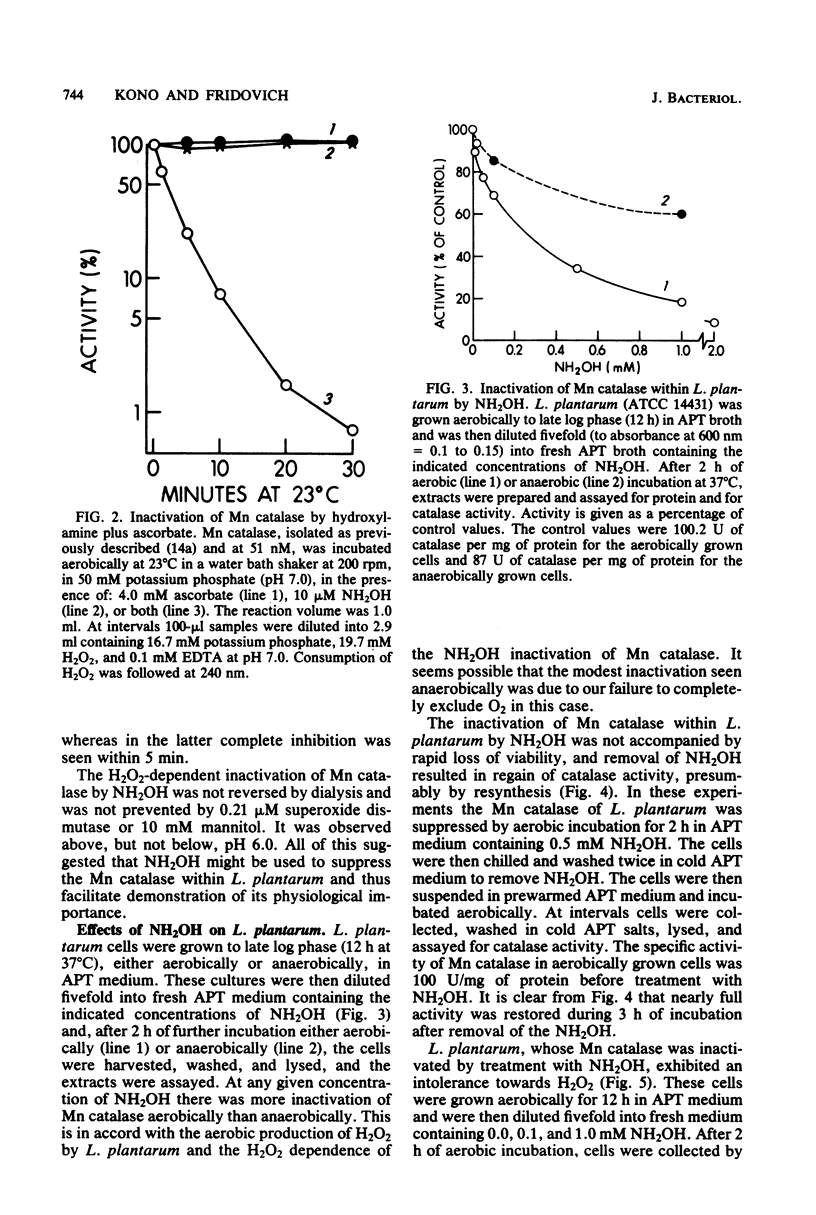
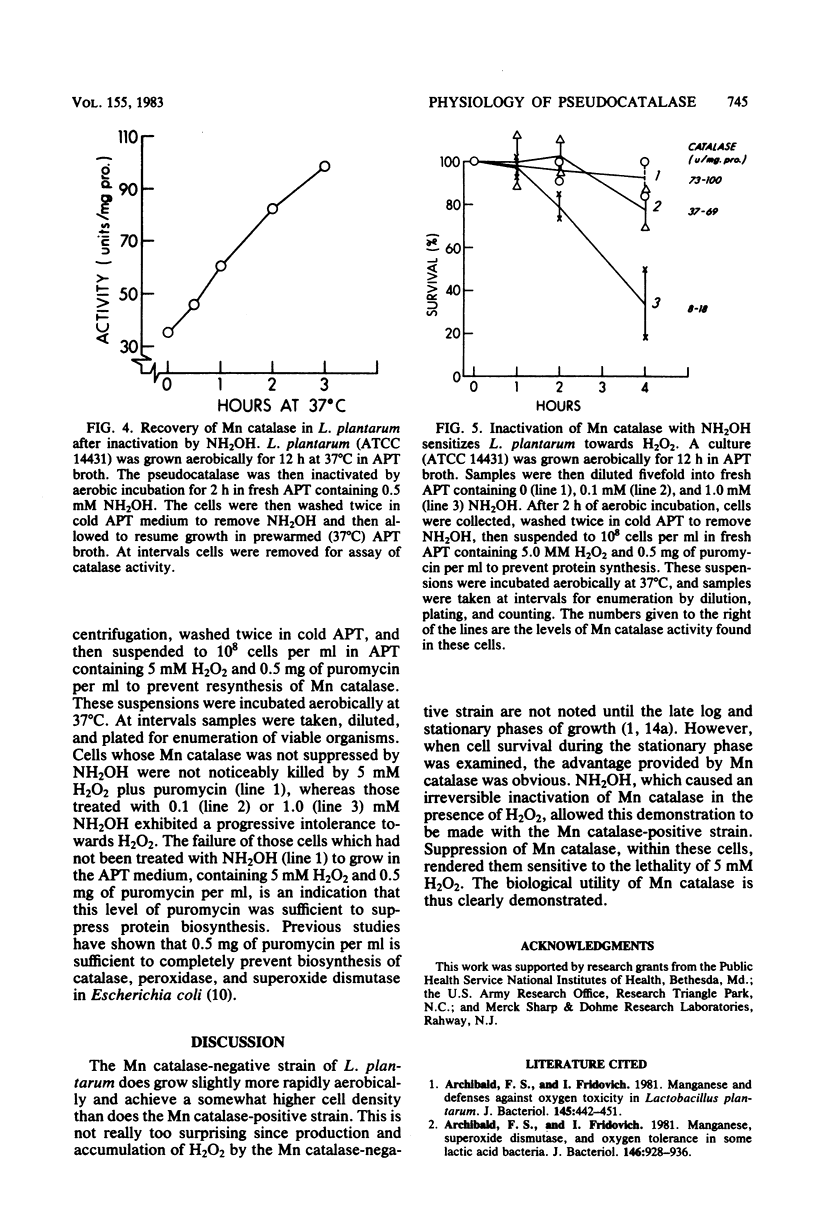
A strain of Lactobacillus plantarum which was unable to produce manganese (Mn)catalase (ATCC 8014) grew somewhat more rapidly and to a slightly higher plateau density than did an Mn catalase-positive strain (ATCC 14421), and this was the case during aerobic or anaerobic growth. However, when maintenance of viability was measured during the stationary phase of the growth cycle, the advantage provided by Mn catalase was obvious. Thus, the viability of ATCC 14431 was undiminished over 21 h of aerobic incubation, during the stationary phase, whereas that of ATCC 8014 decreased by seven orders of magnitude. Addition of catalase to the medium or growth in the presence of hemin, which allows catalase synthesis, protected ATCC 8014 against this loss of viability. Suppression of Mn catalase within ATCC 14431 by treatment with NH2OH caused the cells to lose viability when exposed to 4 mM H2O2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Fridovich I. Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys. 1982 May;215(2):589–596. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981 Jan;145(1):442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981 Jun;146(3):928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELWICHE E. A. Catalase of Pedicoccus cerevisiae. J Bacteriol. 1961 Mar;81:416–418. doi: 10.1128/jb.81.3.416-418.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J., STONE R. W. Oxidative metabolism in Pediococcus pentosaceus. I. Role of oxygen and catalase. J Bacteriol. 1962 Oct;84:716–723. doi: 10.1128/jb.84.4.716-723.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS J. B., NIVEN C. F., Jr Nutrition of the heterofermentative Lactobacilli that cause greening of cured meat products. J Bacteriol. 1951 Nov;62(5):599–603. doi: 10.1128/jb.62.5.599-603.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. Catalase of the Lacto-bacillaceae. J Bacteriol. 1962 Apr;83:936–938. doi: 10.1128/jb.83.4.936-938.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. DISTRIBUTION AND CHARACTERISTICS OF THE CATALASES OF LACTOBACILLACEAE. J Bacteriol. 1965 Aug;90:347–351. doi: 10.1128/jb.90.2.347-351.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. ISOLATION AND CHARACTERIZATION OF THE CYANIDE-RESISTANT AND AZIDE-RESISTANT CATALASE OF LACTOBACILLUS PLANTARUM. J Bacteriol. 1965 Aug;90:352–356. doi: 10.1128/jb.90.2.352-356.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES D., DEIBEL R. H., NIVEN C. F., Jr CATALASE ACTIVITY OF TWO STREPTOCOCCUS FAECALIS STRAINS AND ITS ENHANCEMENT BY AEROBIOSIS AND ADDED CATIONS. J Bacteriol. 1964 Sep;88:602–610. doi: 10.1128/jb.88.3.602-610.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983 May 25;258(10):6015–6019. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Johnson J. L., Salin M. Oxygen metabolism of catalase-negative and catalase-positive strains of Lactobacillus plantarum. J Bacteriol. 1975 Jul;123(1):242–247. doi: 10.1128/jb.123.1.242-247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



