Abstract
Ras-GRF1 has been implicated as a Ras-specific guanine nucleotide exchange factor (GEF), which mediates calcium- and muscarinic receptor-triggered signals in the brain. Although a Dbl homology domain known as a motif conserved among GEFs that target Rho family GTP-binding proteins exists in Ras-GRF1, GEF activity toward Rho family proteins has not been observed. Here we show that Ras-GRF1 exhibits Rac1-specific GEF activity when recovered from cells overexpressing G protein βγ subunits (Gβγ). Substitution of conserved amino acids within the Dbl homology domain abolished this activity. Activation of the Rac pathway in the cell was further evidenced by synergistic activation of the stress kinase JNK1 by Ras-GRF1 and Gβγ, which is sensitive to inhibitory action of dominant-negative Rac1(17N). In addition, association of Ras-GRF1 with Rac1(17N) was demonstrated by coimmunoprecipitation. Evidence for the involvement of tyrosine kinase(s) in Gβγ-mediated induction of Rac1-specific GEF activity was provided by the use of specific inhibitors. These results suggest a role of Ras-GRF1 for regulating Rac-dependent as well as Ras-dependent signaling pathways, particularly in the brain functions.
Keywords: Dbl, guanine nucleotide exchange factor
In mammalian cells, GTP-binding proteins direct diverse intracellular events associated with cell growth, differentiation, and transformation (1). The activation of GTP-binding proteins occurs through the exchange of bound GDP and GTP, which is regulated by guanine nucleotide exchange factors (GEFs) specific to each member.
The Ras-GEF family consists of at least five members, including mSos, Ras-GRF/CDC25Mm, and Ras-GRP. A role for Sos proteins (mSos1 and 2) in tyrosine kinase-type, cytokine, and G protein-coupled receptors has been investigated extensively, leading to the notion that they act downstream of tyrosine kinases and adaptor proteins (2, 3). Ras-GRF1/CDC25Mm (4–7) is expressed exclusively in the brain, while a close relative of Ras-GRF1, Ras-GRF2 (8), is expressed not only in the brain, but also in several other tissues, including lung and spleen, suggesting distinct roles of the two molecules. Ras-GRP (9) is detected in the nervous system, where it may be involved in calcium and diacylglycerol regulation of Ras.
Ras-GRF1 has been shown to be responsible for Ras activation in response to Ca2+ influx (10). This activation is mediated by calmodulin that binds to an IQ motif between the amino-terminal pleckstrin homology (PH) and Dbl homology (DH) domains. On the other hand, it has been demonstrated that stimulation of muscarinic receptors or expression of G protein βγ subunits (Gβγ) confer an increased Ras-GDP/GTP exchange activity to Ras-GRF1 in a phosphorylation-dependent manner (11). In either case, Ras-GRF1 is believed to play a significant role in the regulation of brain-specific Ras functions because mice lacking Ras-GRF1 exhibit defects in certain brain functions such as memory consolidation (12).
Like Ras, the GDP/GTP state of the Rho family GTP-binding proteins, including Rho, Rac, and Cdc42, is modulated by their specific GEFs (13). To date, more than 10 proteins were identified as GEF for Rho family members although the role of each GEF in receptor-mediated signaling is virtually unknown. The DH domain, which is conserved among all Rho family-specific GEFs, is required for the GEF activity. The PH domain contiguous to the DH domain is also important for exerting biological functions. Interestingly, the Ras-targeted GEF Ras-GRF1 also contains the tandem DH/PH domains in its amino-terminal portion although no GEF activity toward Rho family members has been reported so far. Recently, Ras-GRF2, which exhibits overall sequence similarity to Ras-GRF1, has been shown to activate both Ras and Rac, thereby regulating both extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) pathways (14). The activation of ERK and JNK through Ras-GRF2 was further enhanced on calcium ionophore treatment, suggesting a role of Ras-GRF2 in mediating calcium signals to Ras and Rac (14). Mammalian Sos proteins also contain the DH/PH domains, whose function recently has been identified as the regulation of GDP/GTP exchange of Rac downstream of the activated form of Ras (15). These findings suggest that GEF activity of Ras-GRF1 toward Rho family members may be latent in unstimulated cells, and additional signals or modifications may be required for Ras-GRF1 to act as a Rho family GEF. Thus, we examined such possible GEF activity of Ras-GRF1 recovered from cells after a variety of stimulations.
In this paper, we show that Ras-GRF1 acts as a GEF toward Rac1 in response to signals triggered by Gβγ. Ras-GRF1 immunoprecipitated from human embryonic kidney 293 cells overexpressing Gβγ displayed enhanced GEF activity toward the recombinant Rac1 protein. Further, synergistic activation of the stress-responsive kinase JNK1 by Ras-GRF1 and Gβγ, which is sensitive to the action of dominant-negative Rac1, was observed. These findings provide insights into the understanding of the role and cross-talk of small GTP-binding proteins, particularly Ras and Rac, in the brain.
MATERIALS AND METHODS
Materials.
The tyrosine kinase inhibitor herbimycin A was purchased from Calbiochem. The tyrosine kinase inhibitor PP2 was kindly provided by Alexander Levitzki (The Hebrew University of Jerusalem, Jerusalem, Israel).
Cell Culture and Transfection.
The 293 cells were maintained in DMEM supplemented with 10% (vol/vol) FBS. Transfection of 293 cells with expression plasmids was carried out as described (16). For starvation, cells were incubated in DMEM supplemented with 1 mg/ml BSA for 24 h.
Plasmid Construction.
For expression of glutathione S-transferase (GST)-fusion proteins in Escherichia coli, cDNAs encoding Rac1, RhoA, and Cdc42 were subcloned into pGEX-2T (Amersham Pharmacia). The rat Ras-GRF1 cDNA (kindly provided by Larry Feig, Tufts University School of Medicine, Boston, MA) (5) was subcloned into the mammalian expression vector pCMV5 (17), generating pCMV5-Ras-GRF. The cDNA encoding Ras-GRF1 tagged with a FLAG-kinker sequence (MDYKDDDDKLIPGISGGGGG) was constructed by PCR and subcloned into pCMV5 to generate pCMV5-FLAG-Ras-GRF. Plasmids encoding Ras-GRF1 mutants that contain a mutated DH domain (390LTLHELL396 is replaced with 390IIIRDII396) or a deletion of amino-terminal 129 amino acids [pCMV5-FLAG-Ras-GRF(DH(−)) and pCMV5-FLAG-Ras-GRF(ΔN129), respectively] were constructed through sequential PCR steps. cDNAs encoding FLAG-tagged dominant-negative mutants of small GTP-binding proteins [FHa-Ras(17N), FRac1(17N), FRhoA(19N), and FCdc42(17N)] also were subcloned into pCMV5. Expression plasmids for Gαo1, Gβ1, Gγ2, and the muscarinic m2 receptor (pCMV5-αo1, pCMV5-β1, pCMV5-γ2, and pCMV5-m2R, respectively) have been described (18, 19). All PCR products were sequenced to confirm the primary structure of encoded proteins.
Purification of Recombinant GTP-Binding Proteins.
The Rho family proteins Rac1, RhoA, and Cdc42 were purified first as a GST-fusion protein followed by the removal of the GST moiety. The E. coli strain DH5α (for Rac1 and RhoA) or BL21 (for Cdc42) carrying the expression plasmid for each GST-fusion protein was cultivated in the presence of 0.5 mM isopropyl 1-thio-β-d-galactoside at 25°C overnight. Harvested cells were disrupted by sonication in E. coli lysis buffer [50 mM Tris⋅HCl, pH 7.5/5 mM MgCl2/2 mM DTT/1 mM EDTA/1% (wt/vol) sodium cholate/2 μg/ml aprotinin/10 μg/ml leupeptin/10 μg/ml pepstatin A/1 mM benzamidine], and centrifuged at 23,000 × g for 30 min. Cell lysates were applied to a glutathione-Sepharose (Amersham Pharmacia) column, which subsequently was washed with GST wash buffer (20 mM Tris⋅HCl, pH 7.5/2 mM MgCl2/1 mM DTT/2 mM EGTA/0.1 μM GDP). GST-fusion proteins were eluted with glutathione buffer (50 mM Tris⋅HCl, pH 8.0/10 mM glutathione/0.1 μM GDP), and dialyzed against dialysis buffer A [20 mM Tris⋅HCl, pH 7.5/2 mM MgCl2/0.1 mM DTT/10% (vol/vol) glycerol]. After adding 1 mM DTT and 0.1 μM GDP, samples were stored at −80°C.
GST-fusion proteins were treated with thrombin (10 units/mg of GST-fusion protein, Sigma) in buffer containing 50 mM Tris⋅HCl (pH 7.5) and 2.5 mM CaCl2 at 4°C overnight, yielding recombinant Rac1, RhoA, and Cdc42 proteins. Rac1 was purified by using a Q-Sepharose (Amersham Pharmacia) column. Thrombin-digested GST-Rac1 was applied to the column equilibrated with buffer A (50 mM Tris⋅HCl, pH 7.5/2 mM MgCl2/1 mM DTT/0.1 μM GDP). Rac1 was eluted by the same buffer as unbound protein fractions. Thrombin was further eliminated by benzamidine-Sepharose (Amersham Pharmacia) in buffer B (50 mM Tris⋅HCl, pH 8.0/2 mM MgCl2/1 mM DTT/150 mM NaCl/0.1 μM GDP), and purified Rac1 was dialyzed against dialysis buffer B [20 mM Hepes-NaOH, pH 7.4/2 mM MgCl2/0.1 mM DTT/10%(vol/vol) glycerol]. After adding 1 mM DTT and 0.1 μM GDP, samples were stored at −80°C. RhoA and Cdc42 were purified by a Mono Q (Amersham Pharmacia) column. Thrombin-digested GST-RhoA or GST-Cdc42 was applied to the column equilibrated with buffer C (40 mM Tris⋅HCl, pH 8.0/2 mM MgCl2/2 mM DTT), which subsequently was developed with a linear gradient of NaCl (0–150 mM for RhoA and 0–250 mM for Cdc42, respectively). Peak fractions were collected, and purified RhoA and Cdc42 proteins were dialyzed and stored in the same manner as described for Rac1.
In Vitro GDP/GTP Exchange Assays.
GDP/GTP exchange assays were performed essentially as described (11). The 293 cells transfected with various combinations of plasmids were serum-starved for 24 h. Cells were rinsed with ice-cold PBS, dissolved in RIPA buffer [50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/1%(vol/vol) NP-40/0.5%(wt/vol) sodium deoxycholate/0.1%(wt/vol) SDS/0.1 mM PMSF/25 μg/ml leupeptin/25 μg/ml aprotinin/1 mM Na3VO4/50 mM NaF/50 mM β-glycerophosphate/20 mM Na4P2O7], and centrifuged at 15,000 × g for 5 min. Subsequently, the anti-FLAG antibody M2 (2 μg, Kodak) and a rabbit anti-mouse antibody conjugated to protein A-Sepharose (Amersham Pharmacia) were added to the cell lysate, followed by incubation for 1 h with gentle mixing. Immunoprecipitates were washed twice with RIPA buffer and twice with exchange buffer (10 mM Hepes-NaOH, pH 7.4/5 mM MgCl2/5 mM KCl/1 mM EGTA) and subjected to GDP/GTP exchange assays.
For [3H]GDP binding assays, immunoprecipitates were incubated at 30°C in exchange buffer supplemented with 200 ng of each small GTP-binding protein, 2 mM DTT, 0.2 mg/ml BSA, 1 mM ATP, and 1 μM [3H]GDP (1,265 TBq/mol). After incubation for specified periods, ice-cold wash buffer (10 mM Tris⋅HCl, pH 7.5/10 mM MgCl2) was added, and the samples were filtered through the nitrocellulose membrane, which subsequently was subjected to extensive washing with wash buffer. The radioactivity remained on the filter was quantitated by the liquid scintillation counter.
For [3H]GDP release assays, the small GTP-binding protein⋅[3H]GDP complex was prepared by incubation of each small GTP-binding protein with 1 μM [3H]GDP (1,265 TBq/mol) in exchange buffer supplemented with 2 mM DTT, 5 mM EDTA, 0.2 mg/ml BSA, 1 mM ATP for 90 min at 30°C followed by the addition of 5 mM MgCl2 to terminate the GDP/GTP exchange reaction. Immunoprecipitates were incubated with the small GTP-binding protein⋅[3H]GDP complex at 30°C in exchange buffer supplemented with 2 mM DTT, 0.2 mg/ml BSA, 1 mM ATP, and 10 mM GDP. After incubation for specified periods, the radioactivity remained in the complex was quantitated by filter binding assays as described above.
Protein Kinase Assays.
Expression plasmids encoding hemagglutinin (HA)-tagged JNK1 and HA-tagged ERK2 were kindly provided by Michael Karin (University of California-San Diego, La Jolla, CA). Ectopically expressed HA-JNK1 or HA-ERK2 was immunoprecipitated from 293 cells with 0.2 μg of an anti-HA antibody (Boehringer Mannheim) conjugated to protein A-Sepharose. Kinase assays for JNK1 and ERK2 were carried out as described (20).
Immunoprecipitation and Immunoblotting.
Cells were dissolved in IP buffer [20 mM Tris⋅HCl, pH 7.5/250 mM NaCl/3 mM EDTA/3 mM EGTA/0.5%(vol/vol) NP-40/1 mM PMSF/10 μg/ml leupeptin/0.1 mM Na3VO4/3 mM β-glycerophosphate], and centrifuged at 15,000 × g for 15 min. The anti-FLAG antibody M2 (2 μg) and a rabbit anti-mouse antibody conjugated to protein A-Sepharose were mixed gently with the cell lysate for 2 h at 4°C, and the precipitate was washed four times with IP buffer. The precipitated proteins then were separated by SDS/PAGE and transferred onto the nitrocellulose membrane. The membrane was stained with an anti-Ras-GRF1 or antiphosphotyrosine (PY99) antibody (Santa Cruz Biotechnology) and enhanced chemiluminescence detection reagents (DuPont/NEN).
RESULTS
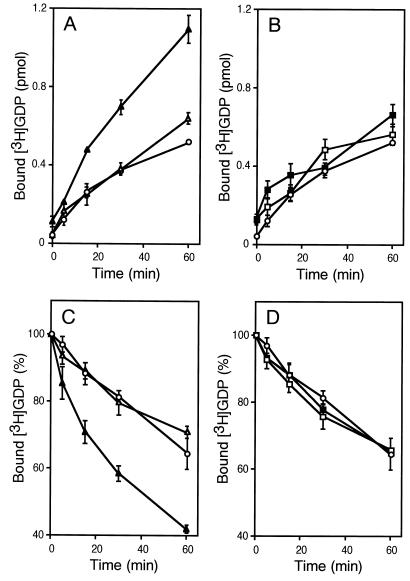
Fig. 1 shows stimulated GEF activity toward Rac1 of Ras-GRF1 obtained from Gβγ-overexpressing 293 cells. The 293 cells were transfected with a plasmid that expresses FLAG-tagged full-length Ras-GRF1 alone or together with plasmids encoding G protein β1 and γ2 subunits, respectively. Expression levels of Ras-GRF1 were virtually identical between samples in each experiment as defined by immunoblotting using the anti-FLAG antibody M2 (data not shown). Subsequently, GEF activity of immunoprecipitated Ras-GRF1 toward the recombinant Rac1 protein was assessed by using filter binding assays. Both the binding of [3H]GDP to Rac1 and the release of [3H]GDP from the Rac1⋅[3H]GDP complex were promoted by the Ras-GRF1 immunoprecipitate from Gβγ-coexpressing cells compared with that recovered from mock-transfected cells (Fig. 1 A and C). In contrast to Ras-GEF activity (11), no Rac-GEF activity was detected unless the cells were cotransfected with Gβγ, consistent with the previous results that recombinant Ras-GRF1 displayed no Rac-GEF activity (5). To ascertain that the DH domain is responsible for Rac-directed GEF activity, we generated a Ras-GRF1 construct [termed Ras-GRF1(DH-)] containing a substitution of highly conserved seven aa within the DH domain (390LTLHELL396 is replaced with 390IIIRDII396) (21). Similar mutation is known to render the Dbl protein incapable of stimulating GDP/GTP exchange of Cdc42 (22) and transformation of NIH 3T3 cells (23). As shown in Fig. 1 B and D, Ras-GRF1(DH(-)) no longer exhibited Rac-GEF activity even when it was immunoprecipitated from Gβγ-overexpressing cells.
Figure 1.
GEF activity of Ras-GRF1 toward Rac1. (A) Effect of wild-type Ras-GRF1 on GDP binding to Rac1. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF alone (▵), or pCMV5-FLAG-Ras-GRF with pCMV5-β1 and pCMV5-γ2 (▴). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP binding assays. Data are shown as mean ± SE (n = 3). (B) Effect of the DH domain mutant Ras-GRF1(DH(-)) on GDP binding to Rac1. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF(DH(-)) alone (□), or pCMV5-FLAG-Ras-GRF(DH(-)) with pCMV5-β1 and pCMV5-γ2 (■). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP binding assays. Data are shown as described in A. (C) Effect of wild-type Ras-GRF1 on GDP release from the Rac1⋅GDP complex. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF alone (▵), or pCMV5-FLAG-Ras-GRF with pCMV5-β1 and pCMV5-γ2 (▴). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP release assays. Relative amounts of [3H]GDP remaining in the complex to the value at 0 min (100% = 1.25 pmol) are shown as mean ± SE (n = 3). (D) Effect of the DH domain mutant Ras-GRF1(DH(-)) on GDP release from the Rac1⋅GDP complex. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF(DH(-)) alone (□), or pCMV5-FLAG-Ras-GRF(DH(-)) with pCMV5-β1 and pCMV5-γ2 (■). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP release assays. Data are shown as described in C.
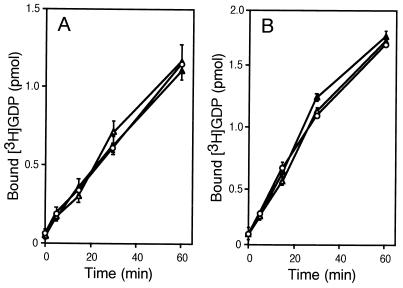
An array of proteins containing the DH/PH domains, including Dbl, Ost, and Trio, target two or three Rho family members, thereby accelerating their GDP/GTP exchange. Thus, we tested GEF activity of Ras-GRF1 toward the other members, RhoA and Cdc42. Under conditions similar to those for detecting Rac-directed GEF activity, no GEF activity toward RhoA and Cdc42 was evident as illustrated in Fig. 2. The lipid-modified form of recombinant RhoA purified from insect cells was also insensitive to Ras-GRF1 (data not shown).
Figure 2.
Effect of Ras-GRF1 on GDP/GTP exchange of RhoA and Cdc42. (A) Effect of wild-type Ras-GRF1 on GDP binding to RhoA. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF alone (▵), or pCMV5-FLAG-Ras-GRF with pCMV5-β1 and pCMV5-γ2 (▴). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP binding assays. Data are shown as described in Fig. 1A. (B) Effect of wild-type Ras-GRF1 on GDP binding to Cdc42. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF alone (▵), or pCMV5-FLAG-Ras-GRF with pCMV5-β1 and pCMV5-γ2 (▴). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP binding assays. Data are shown as described in Fig. 1A.
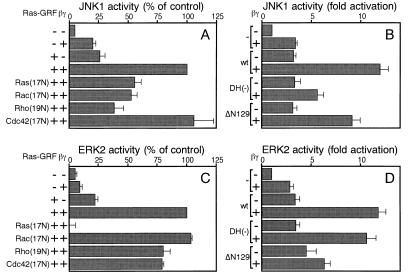
The stress kinase JNK1 is regulated downstream of Rho family members in a variety of cells. Thus, the kinase activity of JNK1 was measured after expression of Ras-GRF1 and Gβγ in 293 cells. As shown in Fig. 3A, overexpressed Gβγ alone caused several-fold stimulation of JNK1 activity as described (24). When ectopically expressed, Ras-GRF1 also augmented JNK1 activity. This effect may be mediated by Ras because Ras-GRF1 alone can activate Ras (5, 10, 11), which in turn triggers JNK1 activation in 293 cells (25). Significantly, concomitant expression of Gβγ and Ras-GRF1 resulted in synergistic activation of JNK1, which is likely to be ascribed to Rac-directed GEF activity of Ras-GRF1 induced by Gβγ as described in Fig. 1. To confirm this hypothesis, we examined the effect of dominant-negative mutants of GTP-binding proteins. Ha-Ras(17N), Rac1(17N), and RhoA(19N) partially inhibited Ras-GRF1 plus Gβγ-induced JNK1 activation, whereas Cdc42(17N) did not exert inhibitory effects. Contrary to the inhibitory action of RhoA(19N), Clostridium botulinum C3 transferase, which specifically inactivates Rho, did not disrupt Gβγ and Ras-GRF1-stimulated JNK1 activation (data not shown). Therefore, Ras and Rac are likely to be responsible for the JNK1 activation. Furthermore, the DH domain mutation that eliminates Rac-GEF activity also attenuated JNK1 activation (Fig. 3B), consistent with the notion that the induced Rac-GEF activity is responsible for the synergistic activation of JNK1.
Figure 3.
Synergistic activation of JNK1 and ERK2 by Ras-GRF1 and Gβγ. (A) Effect of dominant-negative small GTP-binding proteins on Ras-GRF1 and Gβγ-induced JNK1 activation. Activities of HA-tagged JNK1 immunoprecipitated from 293 cells expressing indicated proteins were assessed. Relative values compared with the activity from Ras-GRF1 plus Gβγ-expressing cells are shown as mean ± SE (n = 3). (B) Effect of DH domain mutation and deletion of the amino-terminal PH domain of Ras-GRF1 on Ras-GRF1 and Gβγ-induced JNK1 activation. Activities of HA-tagged JNK1 immunoprecipitated from 293 cells expressing indicated proteins were assessed. Relative values compared with the activity from mock-transfected cells are shown as mean ± SE (n = 3). (C) Effect of dominant-negative small GTP-binding proteins on Ras-GRF1 and Gβγ-induced ERK2 activation. Activities of HA-tagged ERK2 immunoprecipitated from 293 cells expressing indicated proteins were assessed. Data are shown as described in A. (D) Effect of DH domain mutation and deletion of the amino-terminal PH domain of Ras-GRF1 on Ras-GRF1 and Gβγ-induced ERK2 activation. Activities of HA-tagged ERK2 immunoprecipitated from 293 cells expressing indicated proteins were assessed. Data are shown as described in B.
Fig. 3 C and D describes ERK2 activation in response to overexpression of Ras-GRF1 and Gβγ. Like JNK1, ERK2 was remarkably enhanced by concomitant expression of Ras-GRF1 and Gβγ. However, the activation entirely depended on Ras as evidenced by complete inhibition by Ha-Ras(17N) and was insensitive to the inhibitory effects of dominant-negative mutants of Rho family members. Also, the DH mutation showed no negative effect on ERK2 activation in response to Gβγ.
Furthermore, we assessed the role of the amino-terminal PH domain (amino acid 23–129) by the use of the deletion mutant Ras-GRF1(ΔN129). The amino-terminal PH domain cooperatively functions with other motifs including coiled-coil and IQ domains for Ca2+ stimulation of Ras-targeted GEF activity of Ras-GRF1 (26). Although the amino-terminal PH domain was also indispensable for the activation of the Ras/ERK cascade after Gβγ expression (Fig. 3D), it was not absolutely required for Gβγ-triggered JNK activation (Fig. 3B). Hence, the mechanism underlying Gβγ-mediated activation of Rac-GEF activity seems distinct from that of Ca2+- or Gβγ-induced stimulation of Ras-GEF activity.
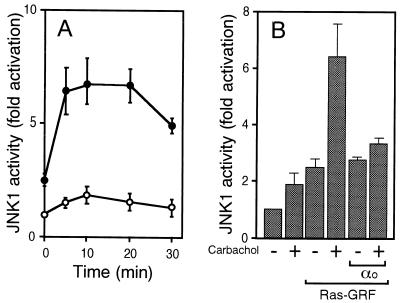
Stimulation of the muscarinic m2 receptor triggers the release of Gβγ, allowing an array of Gβγ-dependent signaling pathways to be activated. Thus, the effect of m2 receptor stimulation on Ras-GRF1-mediated JNK1 activation was examined. As shown in Fig. 4A, JNK1 activation after carbachol treatment was substantially enhanced when Ras-GRF1 was coexpressed. Moreover, the enhanced JNK1 activity was diminished by overexpression of Gαo1, indicative of a role for Gβγ (Fig. 4B).
Figure 4.
Effect of Ras-GRF1 on JNK1 activation in response to m2 receptor stimulation. (A) Time course of JNK1 activation in response to m2 receptor stimulation. Activities of HA-tagged JNK1 immunoprecipitated from 293 cells expressing the m2 receptor alone (○) or the m2 receptor and Ras-GRF1 (●) after stimulation with carbachol (10 μM) for specified periods. Relative values compared with the activity from 293 cells expressing the m2 receptor alone without carbachol stimulation are shown as mean ± SE (n = 3). (B) Effect of Gαo1 on m2 receptor and Ras-GRF1-mediated JNK1 activation. Activities of HA-tagged JNK1 immunoprecipitated from 293 cells expressing the m2 receptor and indicated proteins with (+) or without (−) carbachol stimulation (10 μM, 10 min). Data are shown as described in A.
Fig. 5 shows the association between Ras-GRF1 and dominant-negative forms of small GTP-binding proteins within the cell. Ras-GRF1 was detected in immunoprecipitates of FLAG-tagged Ha-Ras(17N), Rac1(17N), and RhoA(19N), whereas the interaction of Cdc42(17N) with Ras-GRF1 was not observed. Expression levels of small GTP-binding proteins were virtually identical as defined by immunoblotting using the anti-FLAG antibody M2. Also, similar levels of Ras-GRF1 expression in all lysates were confirmed by immunoblotting using an anti-Ras-GRF1 antibody (data not shown). These results are consistent with inhibitory effects of dominant-negative forms of Ha-Ras, Rac1, and RhoA on Ras-GRF1-dependent JNK1 stimulation (Fig. 3A).
Figure 5.
Association of Ras-GRF1 with dominant-negative forms of Ha-Ras, Rac1, and RhoA. Proteins within anti-FLAG antibody M2 (+) or control antibody (−) immunoprecipitates from 293 cells transfected with pCMV5-Ras-GRF and expression plasmids for Gβγ and indicated FLAG-tagged small GTP-binding proteins were stained with an anti-Ras-GRF1 antibody. A representative result of three independent experiments is shown.
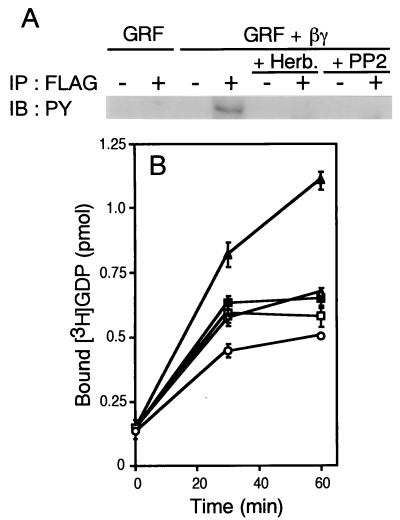
To further understand the mechanisms by which Gβγ stimulates Rac-GEF activity of Ras-GRF1, we investigated tyrosine phosphorylation of this molecule. Immunoprecipitated Ras-GRF1 was tyrosine phosphorylated when recovered from Gβγ-coexpressing cells, as determined by immunoblotting using an antiphosphotyrosine antibody (Fig. 6A). In addition, Gβγ-induced tyrosine phosphorylation of Ras-GRF1 was prevented on treatment with the tyrosine kinase inhibitors herbimycin A and PP2 (Fig. 6A). As shown in Fig. 6B, these inhibitors also impaired Gβγ-stimulated Rac-GEF activity, suggesting that tyrosine phosphorylation in response to Gβγ is crucial for the Rac-GEF activity.
Figure 6.
Involvement of tyrosine kinase(s) in Gβγ induction of Rac-GEF activity of Ras-GRF1. (A) Tyrosine-phosphorylation of Ras-GRF1 on Gβγ expression. Proteins within anti-FLAG antibody M2 (+) or control antibody (−) immunoprecipitates from 293 cells transfected with pCMV5-FLAG-Ras-GRF alone or pCMV5-FLAG-Ras-GRF and expression plasmids for Gβγ were stained with an antiphosphotyrosine antibody. Where indicated, cells were treated with herbimycin A (1 μg/ml) or PP2 (30 μM) for 24 h. A representative result of three independent experiments is shown. (B) Effect of tyrosine kinase inhibitors on Rac-GEF activity of Ras-GRF1. The 293 cells were transfected with a control vector (○), pCMV5-FLAG-Ras-GRF alone (▵), or pCMV5-FLAG-Ras-GRF with pCMV5-β1 and pCMV5-γ2 (▴, □, ■). During serum starvation, cells were treated with herbimycin A (1 μg/ml, □), PP2 (30 μM, ■), or control solvent (○, ▵, ▴). Cell lysates were prepared after serum starvation. Anti-FLAG M2 immunoprecipitates were subjected to [3H]GDP binding assays. Data are shown as described in Fig. 1A.
DISCUSSION
Rho family small GTP-binding proteins have been implicated in regulation of a wide variety of cellular events in many types of cells, including fibroblasts, epithelial cells, and neuronal cells (27). Particularly, the role of Rho family proteins in organization of the actin cytoskeleton is revealed in detail. Rho family proteins also are involved in cell cycle progression, transformation, cell motility, and cell–cell interaction. In parallel with these diverse biological functions, Rho family proteins act downstream of several types of receptors, such as tyrosine kinase-type (e.g., epidermal growth factor and hepatocyte growth factor) and G protein-coupled (e.g., lysophosphatidic acid and muscarinic acetylcholine) receptors. However, the mechanisms underlying the activation of Rho family members in response to membrane receptor signals are still obscure, although a large number of GEFs specific to the Rho family have been identified (13).
In the G protein-coupled receptor system linked to the activation of Rho family members, both α and βγ subunits of G proteins are believed to interact with their specific targets, thereby facilitating downstream signaling cascades. As for Gα signaling, JNK activation after expression of an activated mutant of Gα12, for instance, is mediated by Rac, Cdc42 (28–30), and Rho (Motoshi Nagao, Y.K., and Hiroshi Itoh, unpublished work). Recently, the interaction of p115-RhoGEF with Gα12 and Gα13 and mutual regulation of the catalytic activities were reported, providing a significant link between receptor-coupled G protein and the small GTP-binding protein Rho (31, 32). However, Gβγ-dependent signaling pathways to Rho family proteins remained largely unknown. Our findings, at least in part, account for Gβγ-mediated activation of Rac, although exclusively in the brain. Thus, other GEFs specific to Rho family members may participate in Gβγ-dependent signaling in different cell types.
To date, several examples of signal-dependent stimulation of GEF activity toward Rho family proteins have been described. First, the protooncogene product Vav, which is primarily expressed in hematopoietic cells, is a tyrosine kinase-stimulated GEF for Rac1 (33, 34). Second, mammalian Sos, originally identified as a GEF specific to Ras, shows activated Ras-dependent Rac-GEF activity (15). Herein, we describe another example, Rac1-specific GEF activity of Ras-GRF1 that was recovered from Gβγ-overexpressing cells.
Through the use of specific inhibitors, we have demonstrated that tyrosine phosphorylation is indispensable for the induction of Rac-GEF activity of Ras-GRF1 (Fig. 6). The mechanism is similar to Vav, which displays Rac-targeted GEF activity on phosphorylation by the tyrosine kinase Lck (33, 34). Although largely unknown at present, tyrosine kinases that act downstream of Gβγ, such as Btk family kinases (35) and Src (36) may play a role for the induction of Rac-GEF activity of Ras-GRF1. Given that the Gβγ-stimulated increase of Ras-GEF activity of Ras-GRF1 depends on serine/threonine phosphorylation (11), Rac-GEF activity may be induced through serine/threonine phosphorylation as well. Similarly, threonine phosphorylation of the Rac1-specific GEF Tiam1 may be required for the regulation of its GEF activity because it becomes threonine phosphorylated on stimulation with G protein-coupled receptor ligands, such as lysophosphatidic acid, bombesin, and bradykinin, in a protein kinase C-dependent manner (37).
Ras-GRF1 leads to Ras activation in response to Ca2+ influx through the binding of calmodulin to the IQ motif (10). For this function, the DH/PH domains and the amino-terminal PH domain are required (21, 26). Rac-GEF activity of Ras-GRF2 may be regulated by similar mechanisms because Ras-GRF2-induced JNK activation, which is mediated through Ras and Rac, is enhanced by Ca2+ influx (14). In contrast, the amino-terminal PH domain is not crucial for Gβγ and Ras-GRF1-triggered JNK activation (Fig. 3B), leading to the possibility that the Ca2+/calmodulin signal does not function between Gβγ and Ras-GRF1 to stimulate the Rac pathway.
Conformational change of Ras-GRF1 in response to direct interaction with Gβγ also may be responsible for the induction of Rac-GEF activity because an array of molecules, including adenylyl cyclases, phospholipase Cβ, and a subset of K+ channels, are regulated through direct binding of Gβγ. In fact, binding of Gβγ to the GST-fusion protein containing the PH domain of Ras-GRF1 was reported (38). Yet, arguing against this possibility, we have not been successful in detecting Gβγ associated with immunoprecipitated Ras-GRF1 that exhibits Rac1-directed GEF activity (unpublished results).
In addition to the overexpression of Gβγ, stimulation of the G protein-coupled m2 receptor caused enhanced JNK1 activation in the presence of Ras-GRF1 (Fig. 4A). The enhancement was sensitive to Gαo that sequesters Gβγ, indicative of a role of Gβγ (Fig. 4B). Thus, Ras-GRF1 activation may occur in response to Gβγ released on physiological stimulation also in the nervous system.
In neuronal cells, Rho family proteins are implicated in the process of axonal extension, particularly in the regulation of filopodial and lamellipodial structures in the growth cone (39, 40). However, the role of Rho family proteins in signal transduction downstream of a wide range of G protein-coupled receptors, including neurotransmitter receptors, are virtually unclear. Our present data may provide insights into the brain-specific role of Rac and mechanisms governing pathways coupling Rac and G protein-coupled receptors. Notably, mice lacking Ras-GRF1 are impaired in the process of memory consolidation, while learning and short-term memory are intact (12). Given the results described in this paper, not only Ras, but also Rac may serve as a key element leading to the formation of long-term memories.
Acknowledgments
We are grateful to Larry Feig and Michael Karin for plasmids harboring Ras-GRF1, HA-JNK1, and HA-ERK2, respectively, and Alexander Levitzki for PP2. We also thank Hiroshi Koide for his advice on protein purification. This work was supported in part by CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation. Our laboratory at Tokyo Institute of Technology is supported by the Shering-Plough Corporation.
ABBREVIATIONS
- DH
Dbl homology
- Gβγ
G protein βγ subunits
- GEF
guanine nucleotide exchange factor
- GST
glutathione S-transferase
- PH
pleckstrin homology
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- HA
hemagglutinin
References
- 1.Kaziro Y, Itoh H, Kozasa T, Nakafuku M, Satoh T. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Trends Biochem Sci. 1993;18:273–275. doi: 10.1016/0968-0004(93)90031-h. [DOI] [PubMed] [Google Scholar]
- 3.Pawson T. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 4.Martegani E, Vanoni M, Zippel R, Coccetti P, Brambilla R, Ferrari C, Sturani E, Alberghina L. EMBO J. 1992;11:2151–2157. doi: 10.1002/j.1460-2075.1992.tb05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shou C, Farnsworth C L, Neel B G, Feig L A. Nature (London) 1992;358:351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Mosteller R D, Sanyal P, Gonzales E, McKinney D, Dasgupta C, Li P, Liu B, Broek D. Proc Natl Acad Sci USA. 1992;89:7100–7104. doi: 10.1073/pnas.89.15.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cen H, Papageorge A G, Zippel R, Lowy D R, Zhang K. EMBO J. 1992;11:4007–4015. doi: 10.1002/j.1460-2075.1992.tb05494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fam N P, Fan W, Wang Z, Zhang L, Chen H, Moran M F. Mol Cell Biol. 1997;17:1396–1406. doi: 10.1128/mcb.17.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebinu J O, Bottorff D A, Chan E Y W, Stang S L, Dunn R J, Stone J C. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 10.Farnsworth C L, Freshney N W, Rosen L B, Ghosh A, Greenberg M E, Feig L A. Nature (London) 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 11.Mattingly R R, Macara I G. Nature (London) 1996;382:268–272. doi: 10.1038/382268a0. [DOI] [PubMed] [Google Scholar]
- 12.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance A J, Herron C E, Ramsey M, Wolfer D P, Cestari V, Rossi-Arnaud C, et al. Nature (London) 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 13.Whitehead I P, Campbell S, Rossman K L, Der C J. Biochim Biophys Acta. 1997;1332:F1–F23. doi: 10.1016/s0304-419x(96)00040-6. [DOI] [PubMed] [Google Scholar]
- 14.Fan W, Koch C A, de Hoog C L, Fam N P, Moran M F. Curr Biol. 1998;8:935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- 15.Nimnual A S, Yatsula B A, Bar-Sagi D. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 16.Satoh T, Kato J, Nishida K, Kaziro Y. FEBS Lett. 1996;386:230–234. doi: 10.1016/0014-5793(96)00449-8. [DOI] [PubMed] [Google Scholar]
- 17.Andersson S, Davis D L, Dahlbäck H, Jörnvall H, Russell D W. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 18.Ito A, Satoh T, Kaziro Y, Itoh H. FEBS Lett. 1995;368:183–187. doi: 10.1016/0014-5793(95)00643-n. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi J, Nagao M, Kaziro Y, Itoh H. J Biol Chem. 1997;272:27771–27777. doi: 10.1074/jbc.272.44.27771. [DOI] [PubMed] [Google Scholar]
- 20.Tago K, Kaziro Y, Satoh T. J Biochem. 1998;123:659–667. doi: 10.1093/oxfordjournals.jbchem.a021988. [DOI] [PubMed] [Google Scholar]
- 21.Freshney N W, Goonesekera S D, Feig L A. FEBS Lett. 1997;407:111–115. doi: 10.1016/s0014-5793(97)00309-8. [DOI] [PubMed] [Google Scholar]
- 22.Hart M J, Eva A, Zangrilli D, Aaronson S A, Evans T, Cerione R A, Zheng Y. J Biol Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- 23.Ron D, Zannini M, Lewis M, Wickner R B, Hunt L T, Graziani G, Tronick S R, Aaronson S A, Eva A. New Biol. 1991;3:372–379. [PubMed] [Google Scholar]
- 24.Coso O A, Teramoto H, Simonds W F, Gutkind J S. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 25.Teramoto H, Crespo P, Coso O A, Igishi T, Xu N, Gutkind J S. J Biol Chem. 1996;271:25731–25734. doi: 10.1074/jbc.271.42.25731. [DOI] [PubMed] [Google Scholar]
- 26.Buchsbaum R, Telliez J, Goonesekera S, Feig L A. Mol Cell Biol. 1996;16:4888–4896. doi: 10.1128/mcb.16.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall A. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 28.Collins L R, Minden A, Karin M, Heller Brown J. J Biol Chem. 1996;271:17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- 29.Voyno-Yasenetskaya T A, Faure M P, Ahn N G, Bourne H R. J Biol Chem. 1996;271:21081–21087. doi: 10.1074/jbc.271.35.21081. [DOI] [PubMed] [Google Scholar]
- 30.Mitsui H, Takuwa N, Kurokawa K, Exton J H, Takuwa Y. J Biol Chem. 1997;272:4904–4910. doi: 10.1074/jbc.272.8.4904. [DOI] [PubMed] [Google Scholar]
- 31.Kozasa T, Jiang X, Hart M J, Sternweis P M, Singer W D, Gilman A G, Bollag G, Sternweis P C. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 32.Hart M J, Jiang X, Kozasa T, Roscoe W, Singer W D, Gilman A G, Sternweis P C, Bollag G. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 33.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Nature (London) 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Das B, Wei W, Van Aelst L, Mosteller R D, Khosravi-Far R, Westwick J K, Der C J, Broek D. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langhans-Rajasekaran S A, Wan Y, Huang X. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luttrell L M, Hawes B E, van Biesen T, Luttrell D K, Lansing T J, Lefkowitz R J. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 37.Fleming I N, Elliott C M, Collard J G, Exton J H. J Biol Chem. 1997;272:33105–33110. doi: 10.1074/jbc.272.52.33105. [DOI] [PubMed] [Google Scholar]
- 38.Touhara K, Inglese J, Pitcher J A, Shaw G, Lefkowitz R J. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- 39.Luo L, Hensch T K, Ackerman L, Barbel S, Jan L Y, Jan Y N. Nature (London) 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 40.Kozma R, Sarner S, Ahmed S, Lim L. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]