Abstract
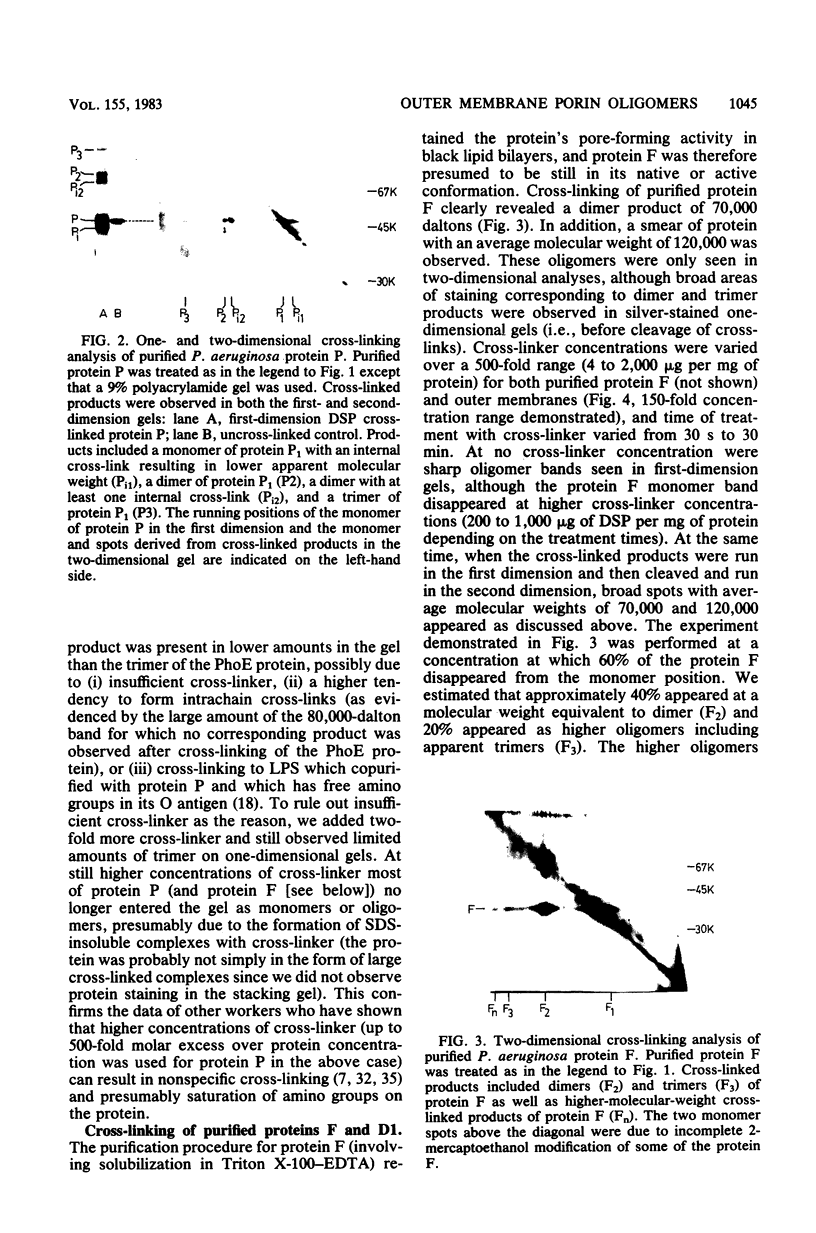
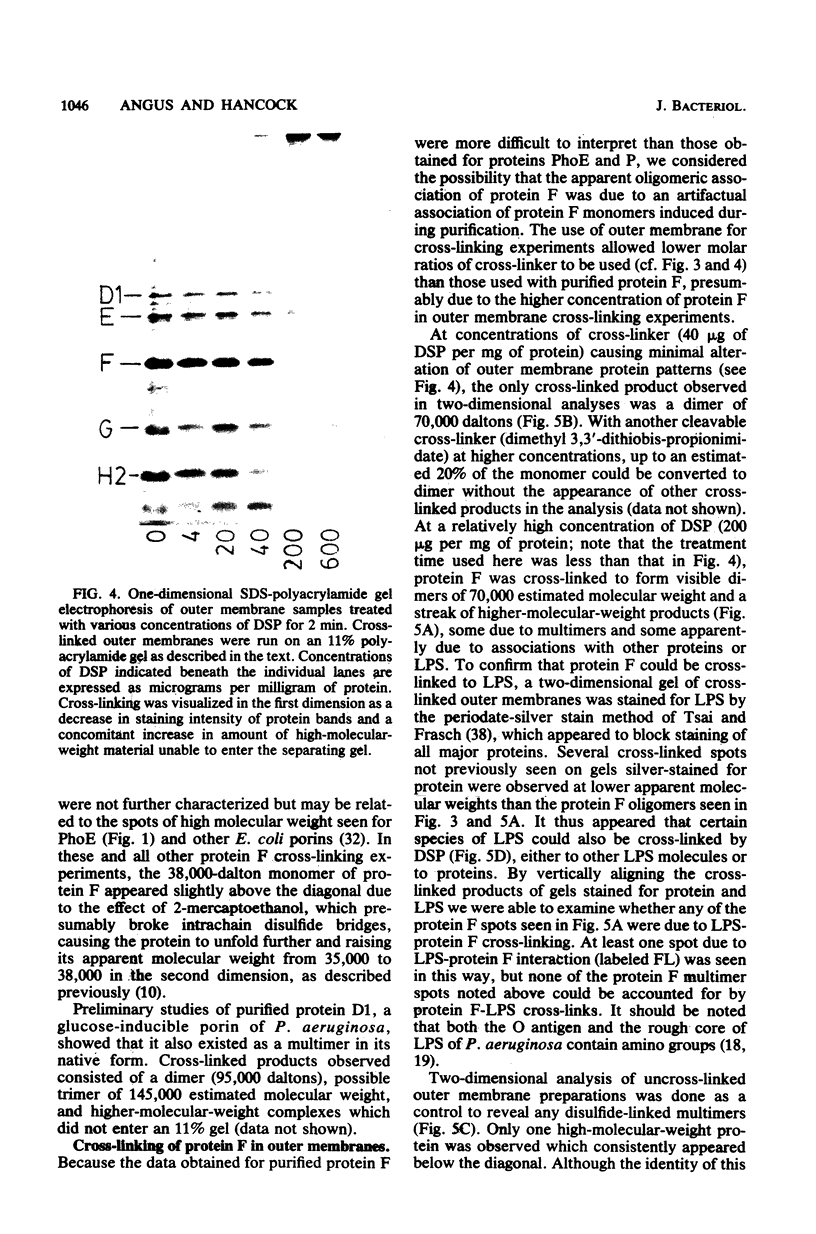
Native oligomers of three Pseudomonas aeruginosa outer membrane porin proteins and one Escherichia coli porin were demonstrated by using a chemical cross-linking technique. P. aeruginosa protein F, the major constitutive outer membrane porin, was cross-linked to dimers in outer membrane and whole-cell cross-linking experiments. Purified preparations of P. aeruginosa proteins F, D1 (glucose induced), and P (phosphate starvation induced) and E. coli protein PhoE (Ic) were also cross-linked to reveal dimers and trimers upon two-dimensional sodium dodecyl sulfate-polyacrylamide electrophoretic analysis. Cross-linking of protein F was abolished by pretreatment of the protein with sodium dodecyl sulfate, indicating that the cross-linked products were due to native associations in the outer membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Boos W. The role of the Escherichia coli lambda receptor in the transport of maltose and maltodextrins. J Supramol Struct. 1980;13(1):101–116. doi: 10.1002/jss.400130110. [DOI] [PubMed] [Google Scholar]
- Foulds J., Chai T. J. New major outer membrane proteins found in an Escherichia coli tolF mutant resistant to bacteriophage TuIb. J Bacteriol. 1978 Mar;133(3):1478–1483. doi: 10.1128/jb.133.3.1478-1483.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Poole K., Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982 May;150(2):730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wieczorek A. A., Mutharia L. M., Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982 Jul;37(1):166–171. doi: 10.1128/iai.37.1.166-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii J., Nakae T. Subunit constituent of the porin trimers that form the permeability channels in the outer membrane of Salmonella typhimurium. J Bacteriol. 1980 Apr;142(1):27–31. doi: 10.1128/jb.142.1.27-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Korteland J., Tommassen J., Lugtenberg B. PhoE protein pore of the outer membrane of Escherichia coli K12 is a particularly efficient channel for organic and inorganic phosphate. Biochim Biophys Acta. 1982 Sep 9;690(2):282–289. doi: 10.1016/0005-2736(82)90332-7. [DOI] [PubMed] [Google Scholar]
- Koval S. F., Meadow P. M. The relationship between aminosurgars in the lipopolysaccharide, serotype, and aeruginocin sensitivity in strains of Pseudomonas aeruginosa. J Gen Microbiol. 1975 Dec;91(2):437–440. doi: 10.1099/00221287-91-2-437. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B. C., Hurlbert R. E. Characterization of the cell wall and cell wall proteins of Chromatium vinosum. J Bacteriol. 1980 Mar;141(3):1386–1398. doi: 10.1128/jb.141.3.1386-1398.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith D. K., Morse S. A. Cross-linking analysis of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1980 Jul;143(1):182–187. doi: 10.1128/jb.143.1.182-187.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Expression of outer membrane protein e of Escherichia coli K12 by phosphate limitation. FEBS Lett. 1980 Apr 7;112(2):229–232. doi: 10.1016/0014-5793(80)80186-4. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Protein interactions in the outer membrane of Escherichia coli. Eur J Biochem. 1979 Feb 1;93(3):495–503. doi: 10.1111/j.1432-1033.1979.tb12848.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Protein neighborhoods in the outer membrane of Salmonella typhimurium. Biochim Biophys Acta. 1980 Feb 28;596(2):235–247. doi: 10.1016/0005-2736(80)90358-2. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Randall L. L. Arrangement of protein I in Escherichia coli outer membrane: cross-linking study. J Bacteriol. 1978 Jan;133(1):279–286. doi: 10.1128/jb.133.1.279-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Randall L. L. Nearest-neighbor analysis of Escherichia coli outer membrane proteins using cleavable cross-links. J Bacteriol. 1976 Sep;127(3):1558–1560. doi: 10.1128/jb.127.3.1558-1560.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Westermann P. Arrangement of the maltose-inducible major outer membrane proteins, the bacteriophage lambda receptor in Escherichia coli and the 44 K protein in Salmonella typhimurium. FEBS Lett. 1979 Mar 1;99(1):77–80. doi: 10.1016/0014-5793(79)80253-7. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]
- Yu F., Ichihara S., Mizushima S. A major outer membrane protein (O-8) of Escherichia coli K-12 exists as a trimer in sodium dodecyl sulfate solution. FEBS Lett. 1979 Apr 1;100(1):71–74. doi: 10.1016/0014-5793(79)81133-3. [DOI] [PubMed] [Google Scholar]