Abstract
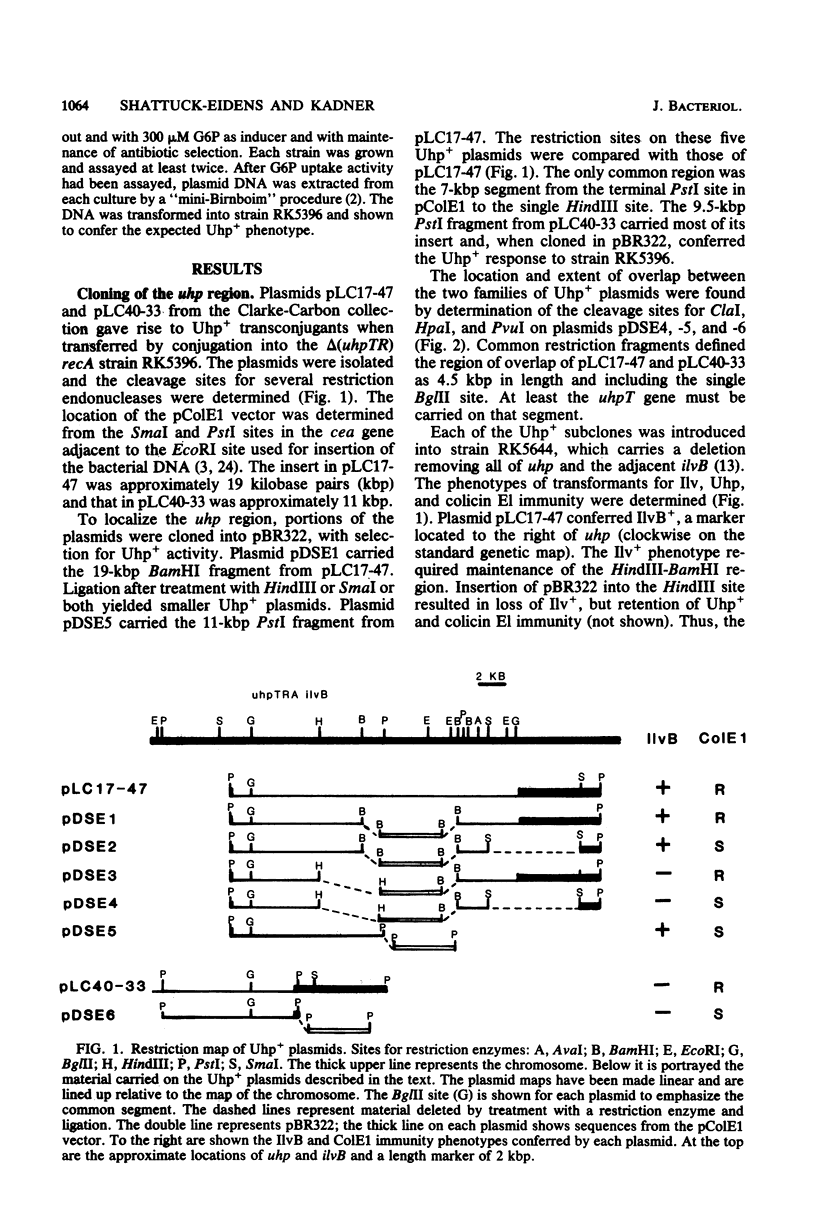
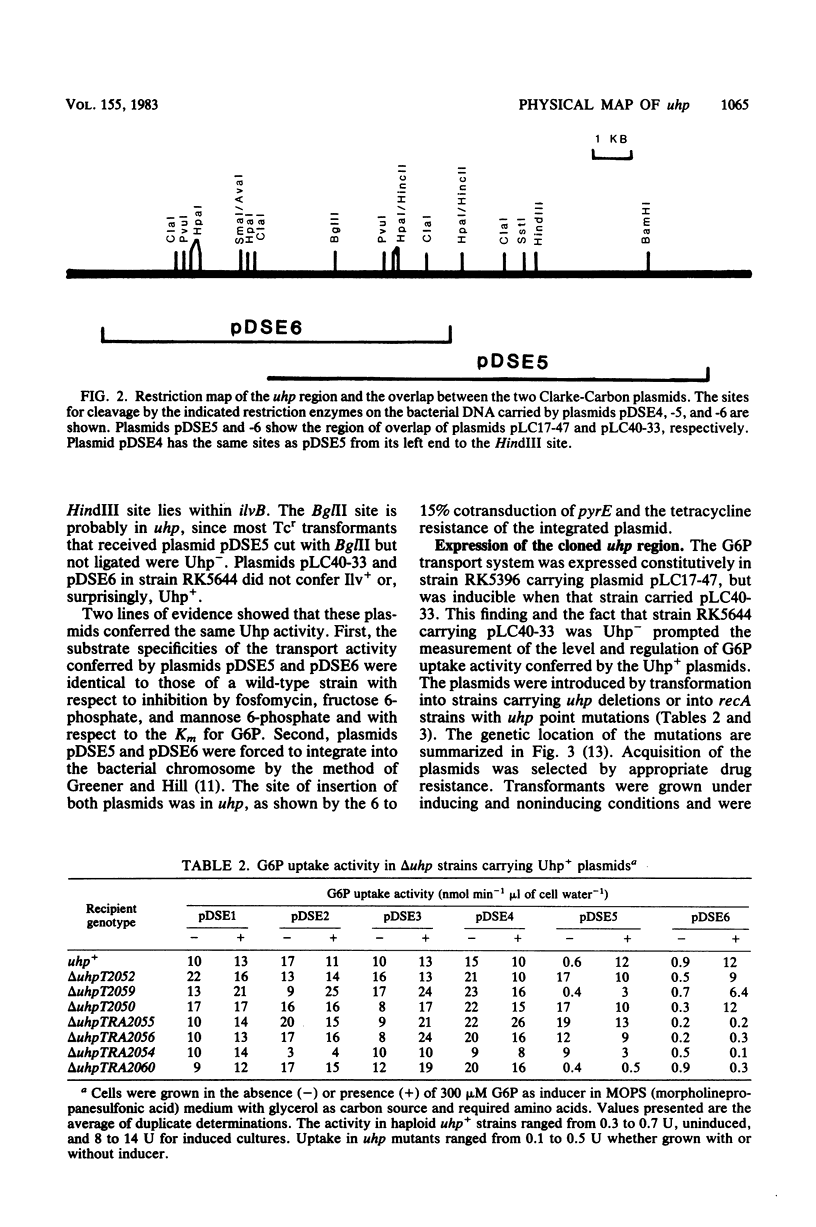
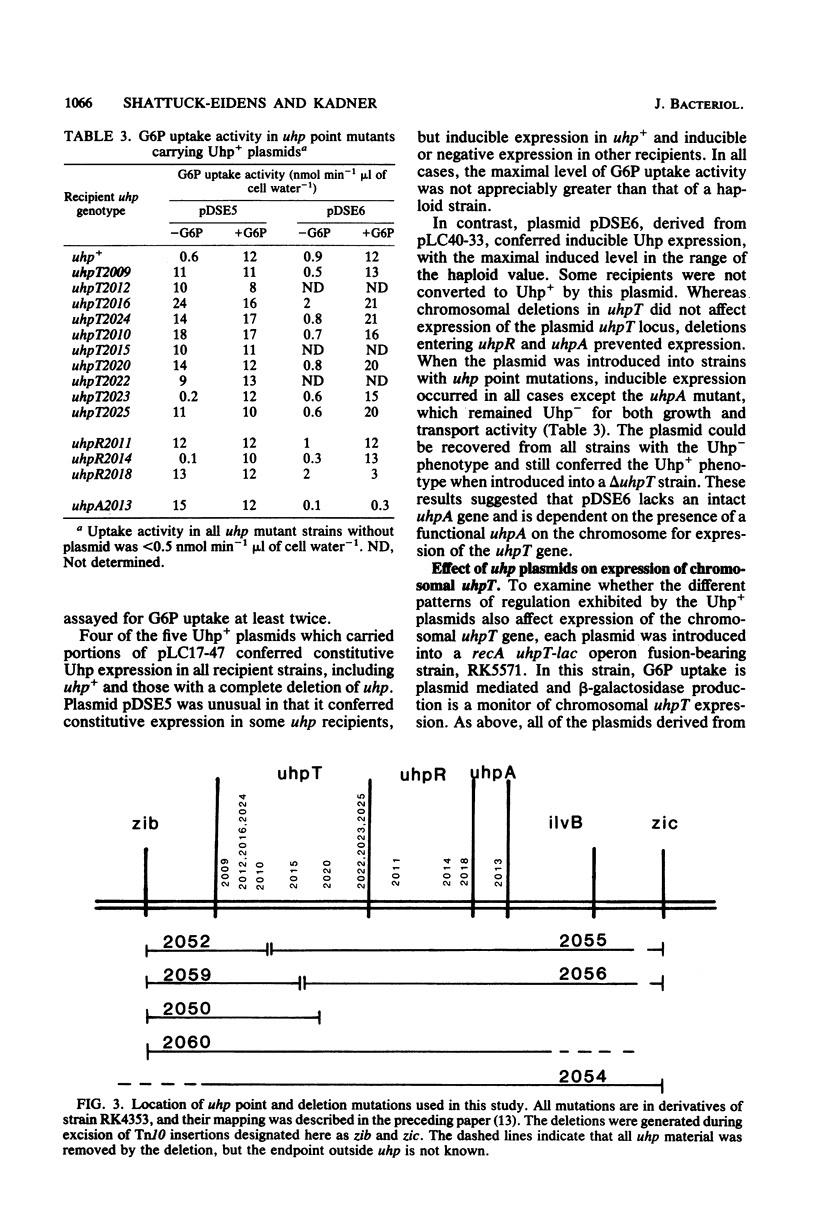
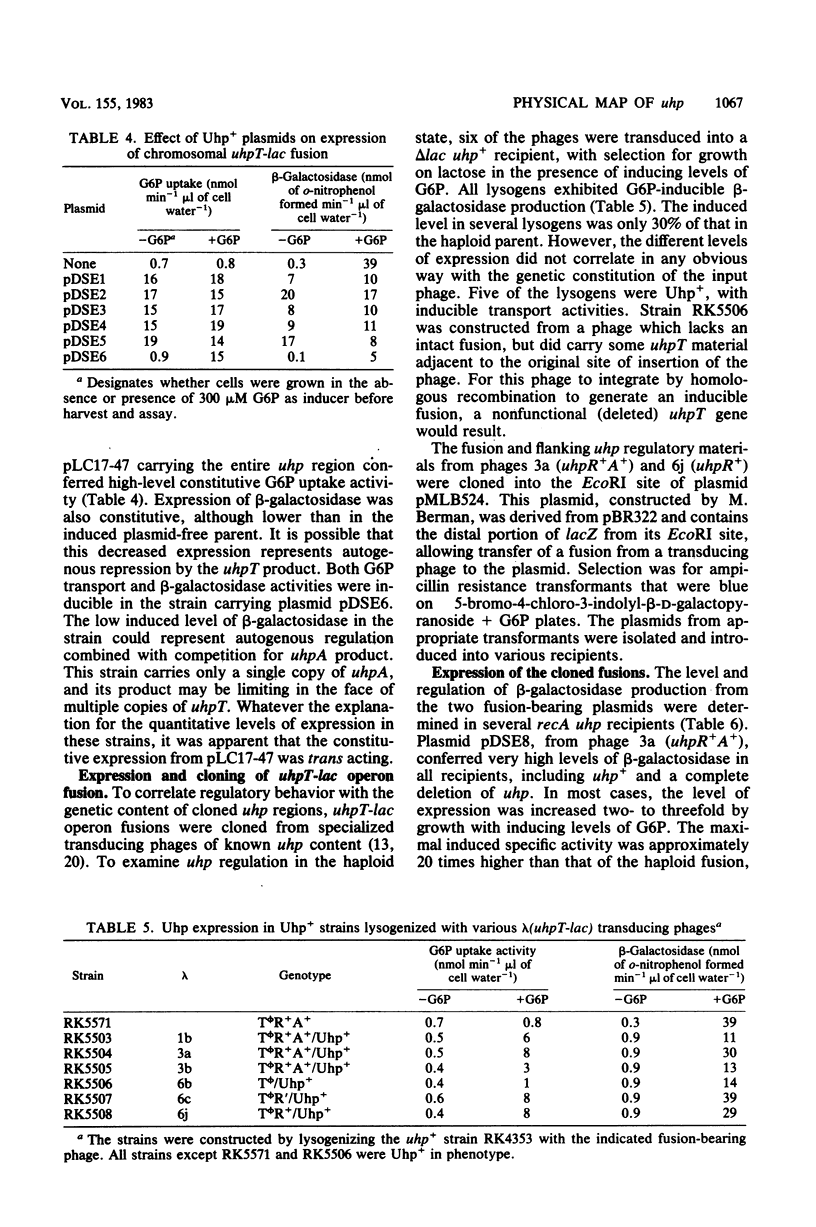
The uhp locus of Escherichia coli contains genes for the sugar phosphate transport system (uhpT) and the regulatory system which allows its induction by external glucose 6-phosphate (uhpRA). The uhp region was cloned onto high-copy-number plasmids, both from Uhp+ plasmids of the Clarke-Carbon collection and from genetically characterized specialized transducing phages carrying uhpT-lac operon fusions. Two Clarke-Carbon plasmids and their Uhp+ subclones in pBR322 shared restriction sites defining the uhp region, but exhibited different regulation of Uhp expression and dependence on chromosomal uhp genotype. Plasmid pLC17-47 and derivatives conferred constitutive glucose 6-phosphate uptake activity in all strains, even those with complete deletions of uhp. These plasmids also rendered constitutive the expression of a chromosomal uhpT-lac operon fusion. Plasmid pLC40-33 conferred inducible Uhp expression, which required the presence of the uhpA+ gene on the chromosome. The induced transport levels in all strains carrying these plasmids were not appreciably amplified over haploid levels. Similar behavior was seen with the cloned operon fusions. A fusion-bearing plasmid that carried an intact regulatory system (uhpR+A+) exhibited trans-dominant constitutive expression of β-galactosidase, regardless of the chromosomal uhp genotype. In contrast, the cloned fusion carrying only uhpR+ gave glucose 6-phosphate-inducible production of β-galactosidase that was dependent on the presence of chromosomal uhpA+. Expression of both fusions in the haploid state was inducible. From these results, it was concluded that the uhpA product is necessary for uhpT transcription and that elevated dosage of uhpA results in at least partially constitutive expression of uhpT. A tentative model for uhp regulation is presented.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Biochemical construction and selection of hybrid plasmids containing specific segments of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4361–4365. doi: 10.1073/pnas.72.11.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essenberg R. C., Kornberg H. L. Location of the gene specifying hexose phosphate transport (uhp) on the chromosome of Escherichia coli. J Gen Microbiol. 1977 Mar;99(1):157–169. doi: 10.1099/00221287-99-1-157. [DOI] [PubMed] [Google Scholar]
- Goldenbaum P. E., Farmer K. S. uhp-directed, glucose 6-phosphate membrane receptor in Escherichia coli. J Bacteriol. 1980 Apr;142(1):347–349. doi: 10.1128/jb.142.1.347-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Genetic Control of the Transport of Hexose Phosphates in Escherichia coli: Mapping of the uhp Locus. J Bacteriol. 1973 Nov;116(2):764–770. doi: 10.1128/jb.116.2.764-770.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Shattuck-Eidens D. M. Genetic control of the hexose phosphate transport system of Escherichia coli: mapping of deletion and insertion mutations in the uhp region. J Bacteriol. 1983 Sep;155(3):1052–1061. doi: 10.1128/jb.155.3.1052-1061.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973 Feb;113(2):895–900. doi: 10.1128/jb.113.2.895-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers T. D., Noti J., Umbarger H. E. Physical characterization of ilv-lac fusions. J Bacteriol. 1979 Oct;140(1):251–260. doi: 10.1128/jb.140.1.251-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Jacobson G. R., Saier M. H., Jr Plasmid-directed synthesis of enzymes required for D-mannitol transport and utilization in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7336–7340. doi: 10.1073/pnas.78.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C. Conversion of circular DNA to linear strands for mapping. Methods Enzymol. 1980;65(1):415–426. doi: 10.1016/s0076-6879(80)65052-6. [DOI] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J Bacteriol. 1981 Oct;148(1):203–209. doi: 10.1128/jb.148.1.203-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Tommassen J., de Geus P., Lugtenberg B., Hackett J., Reeves P. Regulation of the pho regulon of Escherichia coli K-12. Cloning of the regulatory genes phoB and phoR and identification of their gene products. J Mol Biol. 1982 May 15;157(2):265–274. doi: 10.1016/0022-2836(82)90233-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Latterell P. Mutants affected in alkaline phosphatase, expression: evidence for multiple positive regulators of the phosphate regulon in Escherichia coli. Genetics. 1980 Oct;96(2):353–366. doi: 10.1093/genetics/96.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]



