Abstract
13C NMR spectra of aqueous solutions of hyaluronan (HA) of high molecular mass, before and after digestion with testicular hyaluronidase, and of hyaluronan methyl ester were obtained at 125.8 MHz. Carbonyl peaks were assigned by using selective decoupling techniques. Spectra of digested and undigested HA showed sharp signals, except for that assigned to the acetamido carbonyl carbon in the high polymer, which was much broadened. The decreased mobility of this C⩵O, thus demonstrated, was caused by restricted rotation. As part of the rigid CO—NH unit, rotation of NH was therefore similarly restricted, probably because of an intermolecular H bond from NH to carboxylate groups on neighbouring HA molecules. This bond was confirmed by comparing esterified HA with unmodified HA. Methyl esterification of carboxylates was accompanied by changes in acetamido C⩵O resonances consistent with increased mobility of CO—NH groups. Ester C⩵O resonances were sharp, proving that they did not participate in sterically restricted structures such as the proposed H bonds involving unesterified carboxylate groups. C⩵O resonances report on the environments and on the interrelationships of amide and carboxylate groups. A detailed structure suggested for high-molecular-mass HA in aqueous solution takes account of NMR and x-ray fiber diffraction data. Antiparallel HA chains overlap in meshworks stabilized by specific H bonds and hydrophobic bonds. This highly cooperative structure, formally equivalent to β-sheets seen in proteins, is not stable in low-molecular-mass HA solution. The results relate to structures proposed for shape modules in extracellular matrix involving chondroitin and keratan sulfates, which resemble HA in their stereochemistry.
Hyaluronan (HA) is a linear polysaccharide consisting of repeating disaccharide units (Fig. 1), that is, produced by almost all members of the animal kingdom as well as by streptococci (for review, see ref. 1). It is the simplest example of the class of anionic glycosaminoglycans, including the chondroitin, heparan and, keratan sulfates. Their disaccharides contain N-acetyl hexosamine linked to either hexuronate or galactose. The hexosamine in HA is d-glucosamine, and the uronate is d-glucuronate. At a more fundamental level, their polymer backbones are chains of disaccharides that are familiar in other contexts (e.g., in plant polysaccharides); modified by substituents such as acetamido and sulfate, or by oxidation to carboxylates. HA is based on cellobiose (the building block of cellulose); chondroitin and keratan sulfates are polylactoses (2), and heparan sulfate is a polymaltose (compare with starch). The configurations in HA, chondroitin, and keratan sulfates are very similar because their glycosidic bonds follow the same scheme, i.e., 1e–4e, 1e–3e, where e denotes the equatorial orientation of the participating hydroxyl groups. Studies on HA, the archetypal member of the group, will provide important insights into the behavior of the more complicated anionic glycosaminoglycans.
Figure 1.
Primary, secondary and tertiary structures in HA solutions. (A) A tetrasaccharide fragment of HA comprising two repeating disaccharides, showing five H bonds that help to maintain the twofold helix (3). The direct H bond between the acetamido NH and carboxylate seen in dimethyl sulfoxide solution is largely replaced in aqueous solution by a water bridge (4). (B) Three HA chains in a proposed tertiary structure, which accommodates available NMR and x-ray findings, i.e., twofold helices with gentle curves in the polymer backbone in two planes at right angles (5, 2) and hydrophobic patches (hatched) on alternate sides of the polymer (7). The vertical dotted lines delineate the sugar units and the arrows at the right and left sides indicate the reducing terminal direction. Only in antiparallel orientation do the gentle curves in the backbones of the participating molecules complement each other so that interactions are optimal. In antiparallel arrays, the acetamido (■ and □) and carboxylate groups (● and ○) are positioned so that H bonds are possible between them as indicated by arrows pointing from the donor to the acceptor groups. The donor (NH) groups have their N—H bond trans to the 2C—H bond in this model (see text for discussion). The open symbols refer to groups on the proximal side of the tape-like molecule, and the closed symbols are on the distal side. Hydrophobic patches (hatched) on neighboring molecules are contiguous, and hydrophobic bonding between them can occur. The H bonds occur in pairs in the proposed structure, with alternating pairs directed in opposite senses (up and down in the diagram). This structure is formally equivalent to that of the β-sheet in proteins, in which pairs of H bonds are disposed in alternate directions (up and down) between antiparallel polypeptide chains. In combination with the hydrophobic patches, these cooperative interactions would enable large numbers of HA molecules to aggregate specifically, opposed by electrostatic repulsion. This situation is similar to that of the DNA double helix, in which repulsive forces are balanced by hydrophobic bonding and H bonding, although the DNA helix is without the ambidexteran property that permits extensive lateral aggregation. This structure is relevant to the proposed aggregates formed between CS and KS as part of the shape modules in extracellular matrix (8). (C) Scheme of overlapping HA molecules that would allow infinite meshworks to form from HA of high molecular mass. Neighboring molecules are antiparallel, interacting at the atomic level as shown in B above.
HA functions in varied biological environments, both mechanical and metabolic (for review, see ref. 1), implying that the rather simple chemical structure has sophisticated possibilities. Indeed, a secondary structure incorporating up to five H bonds per tetrasaccharide unit of HA was indicated by NMR studies in water and in dimethyl sulfoxide solutions (Fig. 1) (3, 4) as well as by x-ray diffraction data (5). This configuration, a tape-like twofold helix, was subsequently shown to be preferred in the other anionic glycosaminoglycans, also (6). The two sides of the HA tape are identical but antiparallel, suggesting the term ambidexteran—able to use both sides equally (7). They all possess a hydrophobic patch of eight or nine CH units, stretching along three neighboring sugar units and present on alternate sides of the tapelike helices. This feature helps to explain the ability of HA to interact with lipids and membranes, and suggests that it might interact with itself, in water (7).
The characteristic properties of high-molecular-mass HA in water have been of great interest to physiologists and biophysicists for decades (for review, see ref. 1). The non-Newtonian and gel-like properties in, e.g., synovial joints and vitreous humor were explained as random coil-entanglement, based on bulk properties of solutions in which very large numbers of molecules collaborated. These interpretations were based on early physical models.
The application of electron microscopy to rotary-shadowed preparations of HA showed that HA did self-aggregate, into strands of a honeycomb meshwork in aqueous solution. The thickness of the strands increased with HA concentration, which implied that aggregation was both reversible and ordered. No ends were seen to any strands of high-molecular-mass HA, although low-molecular-mass HA formed islands of meshworks under similar conditions (9).
Molecular models of these interactions, based on the twofold helix and involving the hydrophobic patches to promote aggregation, incorporated structural details from x-ray diffraction and NMR studies. Providing aggregating molecules were antiparallel, gentle wave-like dispositions of sugar residues in the tapelike structures complemented each other and intermolecular H-bonds were then seen to be possible, between acetamido NH on one chain and carboxylate on the antiparallel neighboring chain (2). This scheme did not explain how large numbers of HA molecules could aggregate to form the meshworks seen by using electron microscopy. Experimental evidence for the existence of the postulated antiparallel aggregates in solution was not available. This paper presents 13C NMR data that proves that acetamido groups are strictly oriented in high-molecular-mass HA solution in a supramolecular organization, putting modeling of the HA solution structure on a secure basis. The resonances of the amide and carboxylate carbonyls are used to report on their environments and on their interrelationships. A large-scale structure is proposed that provides a molecular basis for the extensive meshworks previously observed, e.g., in streptococcal suspensions (10). The results are relevant to the other extracellular matrix anionic glycosaminoglycans, chondroitin and keratan sulfates.
MATERIALS AND METHODS
HA of high molecular mass (≈106) was dissolved at 10 mg⋅ml−1 in deuterium oxide containing 0.29 M NaCl and 0.05 M phosphate buffer (pH 7.4). This was the highest practical concentration because of the high viscosity of high-molecular-mass HA solutions.
Methylation of high-molecular-mass HA carboxylate groups by dimethyl sulfate in bicarbonate buffer to give methyl ester was performed by using the method of Hallen (11). The saponification value was equivalent to ≈70% esterification, in agreement with the content of methyl ester estimated by 1H NMR (data not shown). Solutions were prepared in D2O as described above.
For NMR decoupling experiments to assign the C⩵O resonances from COO− and CONH in HA, a 5 mg⋅ml−1 solution in H2O was digested by ovine testicular hyaluronidase (EC 3.2.1.35, Fluka) in 0.0105 M phosphate buffer at pH 7.4 containing 0.008 M NaCl for 8 h at 37°C. The solution was freeze-dried, redissolved in D2O, freeze-dried a second time, and finally dissolved in D2O at 40 mg⋅ml−1, at final concentrations of 0.08 M pH 7.4 phosphate buffer and 0.26 M NaCl.
13C NMR spectra were obtained at 30°C by using a Varian Unity 500 spectrometer operating at 125.8 MHz. For standard spectra, 40,000–150,000 transients were acquired using an acquisition time of 0.25 s, a flip angle of 45°, no relaxation delay, and broad-band 1H decoupling.
The 13C spectrum of the high-molecular-mass sample (see above) was compared with that of a hyaluronidase-digested sample, prepared as above, finishing with a 10 mg⋅ml−1 solution in the same conditions of pH, salt, and buffer concentration as the undigested high-molecular-mass HA.
A 13C spectrum of the digested sample at 40 mg⋅ml−1 with selective decoupling of the CH3 protons was obtained by using the same acquisition conditions but with continuous-wave 1H decoupling centered on the 1H CH3 peak with a field strength of ≈50 Hz. A spectrum with 1H coupling was obtained by using the gated decoupling technique with an acquisition time of 0.25 s, a flip angle of 75°, and a relaxation delay of 1 s.
Component analysis of the spectra was performed by using manufacturer-supplied software. A Lorentzian line shape was assumed, and the heights, frequencies, and linewidths of each component were adjusted to give optimum fit to the experimental spectrum.
RESULTS
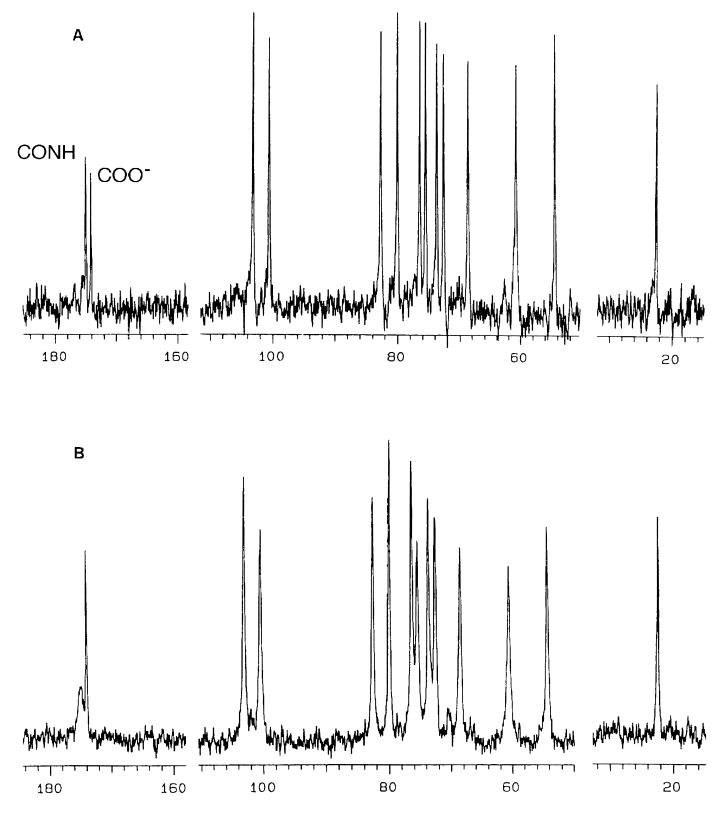
Fig. 2 A and B compare the 13C NMR spectra of digested and undigested HA, respectively, at a concentration of 10 mg⋅ml−1. In spite of the very high viscosity of the undigested compared with the digested sample, both spectra show clearly resolved peaks at the same chemical shifts. Two C⩵O peaks, from the carboxylate and acetamido groups, appeared at 174–175 ppm, two anomeric carbon peaks appeared at 100–104 ppm, the remaining ring carbons appeared at 54–83 ppm, and the acetamido CH3 carbon appeared at 22 ppm. The peaks at 54.5 and 60.5 ppm were assigned, respectively, to the C2 and C6H2OH carbons of the glucosamine residue, based on correspondence of their chemical shifts with similar carbons in mono- and disaccharides (12).
Figure 2.
13C NMR spectra of undigested (A) and digested (B) HA. The concentration was 10 mg⋅ml−1 in each case. See text for discussion. The CONH and COO resonances are labeled, using the assignments derived from the experiment shown in Fig. 3.
In the digested sample, all peaks showed the same natural line width of ≈10–20 Hz. With one exception, the peaks in the undigested sample also showed approximately uniform line-widths of 50–60 Hz, somewhat broader than in the digested sample. The notable exception was the 174.8 ppm C⩵O peak from the undigested sample, which was considerably broader (≈100 Hz) than the other peaks in the same spectra, or any peak in the undigested sample. To understand the structural implications of this broadening, it was necessary to assign the two C⩵O peaks. This was achieved by comparing the fully coupled spectrum with that obtained by using selective decoupling of the CH3 protons (Fig. 3). In the latter, the peak at 174.7 ppm was noticeably narrower than in the former, whereas the peak at 173.9 ppm remained the same width. Thus, the peak at 174.7 ppm, which showed broadening in the spectrum of undigested HA, was assigned to the acetamido carbonyl carbon and that at 173.9 ppm to the carboxylate carbon. Our assignments agree with earlier work (ref. 13 and refs. therein). Broadening of the acetamido carbonyl resonance in high-molecular-mass HA solution, compared with low-molecular-mass HA is also visible in a published spectrum (Fig. 2 of ref. 13), but the authors did not remark upon it.
Figure 3.
Carbonyl 13C spectra of HA, at 40 mg⋅ml−1. (A) coupled spectrum; (B) spectrum with selective low-power decoupling of the CH3 protons. Both spectra were resolution-enhanced using the Lorentz–Gauss transformation with exponential and Gaussian time constants of 0.1 and 0.15 s, respectively. See text for discussion.
The sharpening of the acetamido C⩵O carbon resonance in the digested HA solution implies that the drop in viscosity and molecular mass on digestion frees that C⩵O from restrictions in mobility present in the high-molecular-mass HA solution. The absence of a sharp resonance in the high-molecular-mass acetamido C⩵O signal suggests that a very high proportion of these C⩵O groups are thus restricted. The resonances of the other groups, which can move somewhat independently of the polymer backbone, —CH2OH and —COO− showed no similar broadening, and no comparable changes were observed after digestion, suggesting that neither group is specifically restricted in mobility in the high-viscosity solution.
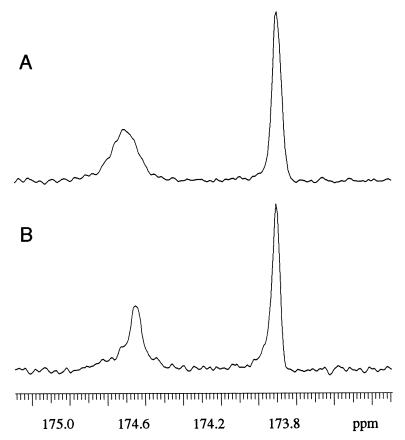
Fig. 4 shows the C⩵O region of the HA methyl ester spectrum. Compared with the original high-molecular-mass HA, the carboxylate C⩵O signal was reduced in intensity, and a sharp methyl ester C⩵O signal appeared at 169.5 ppm. The amide carbonyl peak broadened and became asymmetrical, showing evidence of two peaks, one broad at the same chemical shift as the original HA and the other sharper at ∼0.5 ppm to lower frequency (Fig. 5). This composite structure was confirmed by component analysis of the spectrum of HA methyl ester (Fig. 5B). The sharp peak had about the same chemical shift as that of the sharp amide C⩵O peak in the digested HA.
Figure 4.
Carbonyl region of the 13C NMR spectrum of HA methyl ester.
Figure 5.
Carbonyl region of the 13C NMR spectra of high molecular mass HA samples unmodified (A) and as the methyl ester (B), with component analysis. The smooth line superimposed on the noisy experimental spectrum is the simulated line shape summed from the individual components (C).
DISCUSSION
The presence of a structuring influence in high-molecular-mass HA solution, absent from the same HA after enzymic digestion, suggests that intermolecular interactions are possible and stable in the former solution but absent from the latter. No chemical modification of the fundamental chemical structure would be expected during the digestion, as confirmed by a comparison of the 13C spectra in Fig. 2. A strongly cooperative interaction is implied in the high-molecular-mass HA solution that is not available to the digestion fragments, which are probably mainly tetrasaccharides.
It is instructive to consider the basis of this interaction in the light of the NMR data. It can be assumed that mobility modes open to all other groups (including —CH2OH and —COO−) in the HA molecule because of movement of the chain are also open to the CH3CONH— group. A mode essentially independent of chain mobility is that of rotation, which strongly influences resonance sharpness. The CH3 unit of the acetamido group is free to rotate about the —C—C— bond, as shown by the sharp signal at 22 ppm (Fig. 2). Therefore, the restricted mobility of the acetamido C⩵O group is specific and not a property of the entire acetamido group. However, the only axis of rotation open to the acetamido —C⩵O is that about the —C—N bond. The NH—C⩵O unit is rigid because of mesomerism in which the bonds have partial double bond character. The C⩵O group is thus intrinsically unable to rotate about the —N—C bond. The orientation of this C⩵O is determined by rotation about the —C—N bond at position two of the glucose ring (Fig. 6), which would be as free in the polymer as in the digested fragments unless prevented by interactions not present or stable in the latter.
Figure 6.
Diagrammatic representations of configurations present in solutions of HA methyl ester (a) and high-molecular-mass HA (b). HA1 and HA2 are separate stretches of HA, e.g., on different strands of HA. (a) The ester group rotates freely about the bond to C5 of the glucose ring, as shown by the sharp 13C carbonyl resonance (Fig. 4). This C⩵O therefore does not participate as an acceptor in a H bond similar to that in b, and the amide unit is free to rotate about the N—C2 bond (see Fig. 5 and discussion in text). (b) The orientation of the NH bond and therefore of the C⩵O group is fixed by the H bond to the carboxylate anion. The amide resonance is therefore broad (Figs. 2 and 5). Since there are two equivalent positions in which one of the two oxygen atoms in the carboxylate anion can accept the NH H bond, easy rotation about the C—C5 bond is possible, and the 13C resonance is sharp (Fig. 2B). The arrows in b indicate that HA1 and HA2 are antiparallel.
A H bond from NH to a group on a neighboring molecule would severely restrict rotation about the —C—N— bond. A COO− group was shown by using modeling to be suitably placed in antiparallel HA duplexes to act as acceptor in such a bond (2). Since there are two completely equivalent positions, 180° apart, in which it could accept a proton from the NH, allowing rotation between two positions of equivalent energy, its mobility is much less restricted than that of the H-bonded CO—NH group (Fig. 6). This interpretation allows for the existence of a —NH · · · −O2C— H bond while accounting for the lack of broadening in the —COO− carbon resonance in the solution of high-molecular-mass HA (Fig. 2).
Evidence that the carboxylate is indeed involved in an interaction with the amide NH is provided by the increased complexity and considerable broadening of the amide C⩵O signal in the ester (Fig. 4). No other signal is similarly affected by the esterification, implying that the changes are a direct result of the formation of the ester.
The sharpness of the ester C⩵O signal at 169.5 ppm shows that the group is free to rotate. Thus, it cannot be involved in a tertiary structure that would restrict its rotation. Only one oxygen atom in the ester is available for interactions compared with two in the carboxylate, and any interaction would restrict rotation (Fig. 6). Since the ester carbonyl is therefore not interacting with NH, the change in the amide carbonyl signal is most probably due to a relaxation in the H-bonded structure postulated above, because of replacement of the strongly H-bonding carboxylate with the weaker H-bonding ester (Fig. 6).
Analysis of the complex signal of the amide C⩵O in methylated HA suggested that there were two separate resonances within the observed broad peak (Fig. 5). One, at the same chemical shift as the original HA resonance is accompanied by a new resonance at lower frequency, comparable with that in the digested HA. The two frequencies are thus compatible with H-bonded and a non-H-bonded amide groups, respectively.
The formation of the ester was accompanied by a sharp drop in viscosity of the HA solution, which was not observed in control HA preparations exposed to precisely the same mild reaction conditions but without the alkylating agent (dimethyl sulfate). This result is compatible with the partial or complete disruption of the H-bonded tertiary structure in Fig. 1. Although accurate molecular weights are not available, simple chemical degradation during esterification was unlikely, since the ester was recovered in very high yield, it was nondialyzable, and the 13C NMR spectra showed no signs of chemical modification apart from methylation of the carboxyl group.
Given the robust stability of the glucose C1 form, NH can act as H donor only in a plane roughly at right angles to the sugar ring, which is also at right angles to the plane of the twofold helical polymer. 1H NH/C2H coupling constants of 8–9 Hz in monomers and oligosaccharides (ref. 3, and unpublished observations) imply that the averaged orientations about the CH—NH bond show a preference for the trans configuration, as required in this structure. Although this has not been confirmed in high-viscosity HA solutions, it seems likely. Our observation of restricted C⩵O rotation in high-molecular-mass HA solutions demonstrates that the NH orientation therein is held in a preferred direction. The sugar rings linked by the postulated H bonds would then stack on top of each other, undergoing hydrophobic interactions as proposed previously (2).
This structure is extended in this paper to take in many participating molecules, to account for the branching and honeycomb structures seen by electron microscopy. A series of overlapping interactions in which each HA molecule associates with and binds to antiparallel molecules ahead and behind is proposed as the basis of the gel-like behavior of high-molecular-mass HA solutions (Fig. 1). This tertiary structure is highly demanding, in that it requires that the NH bond is trans to that of the 2C—H bond in a twofold helix in which the pairs of H-bonding groups are, in consequence, trans to each other across the backbone. Each HA molecule thus links to other HA molecules on either side of the HA ambidexteran by secondary valencies. We would point out that this structure is formally equivalent to a β-sheet as seen in many protein structures.
Since there is no evidence of interaction in the digested HA, it follows that there is a minimum size below which cooperative binding between two HA oligosaccharides is not stable. Light-scattering evidence (14) suggested that aggregation of HA oligosaccharides might be possible between oligomers of >20 disaccharides, in salt solutions. Relatively low-molecular-mass HA of >300 disaccharides clearly aggregated, as shown by electron microscopy–rotary shadowing (8).
There may be other interactions (e.g., ref. 15) of lower stability, correspondingly more difficult to demonstrate.
This first proof of a specific stable tertiary cooperative structure in HA solutions is relevant to situations in which anionic glycosaminoglycans such as dermochondan and keratan sulfates aggregate to form the shape modules (8) that help to organize collagen fibrils in extracellular matrices, in which similar antiparallel cooperative structures were proposed.
HA solutions at equilibrium contain a high proportion of ordered structures of the kind in Fig. 1 B and C, some of which would probably be disrupted by mechanical stresses such as pouring, stirring, and in moving synovial joints. The structures would be expected to reform and/or revert on releasing the stress, thus providing the observed elastic bounce that is familiar in mechanically disturbed high-molecular-mass HA solutions.
ABBREVIATION
- HA
hyaluronan
Footnotes
To whom reprint requests should be addressed at: Department of Chemical Morphology, Medical School, Manchester University, Oxford Road, Manchester M13 9PL, United Kingdom.
References
- 1.Laurent T C. The Chemistry, Biology and Medical Applications of Hyaluronan and its Derivatives. London: Portland Press; 1998. [Google Scholar]
- 2.Scott J E. Biochem J. 1994;298:221–222. doi: 10.1042/bj2980221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott J E, Heatley F, Hull W E. Biochem J. 1984;220:197–205. doi: 10.1042/bj2200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heatley F, Scott J E. Biochem J. 1988;254:489–493. doi: 10.1042/bj2540489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins E D T, Meader D, Scott J E. Int J Biol Macromol. 1980;2:318–319. [Google Scholar]
- 6.Scott J E, Heatley F, Wood B. Biochemistry. 1995;34:15467–15474. doi: 10.1021/bi00047a011. [DOI] [PubMed] [Google Scholar]
- 7.Scott J E. FASEB J. 1992;6:2639–2645. [PubMed] [Google Scholar]
- 8.Scott J E, Thomlinson A M. J Anat. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott J E, Cummings C, Brass A, Chen Y. Biochem J. 1991;274:699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott J E. In: The Chemistry, Biology and Medical Applications of Hyaluronan and its Derivatives. Laurent T C, editor. London: Portland Press; 1998. pp. 7–16. [Google Scholar]
- 11.Hallen, A. (1967) Seventh International Congress of Biochemistry, Tokyo, Abstract no. D-53.
- 12.Breitmeier E, Voelter W. Carbon-13 NMR Spectroscopy. High Resolution Methods and Applications in Organic Chemistry and Biochemistry. New York: VCH; 1987. p. 381. [Google Scholar]
- 13.Cowman M K, Hittner D M, Feder-Davis J. Macromolecules. 1996;29:2893–2902. [Google Scholar]
- 14.Turner R E, Lin P, Cowman M. Arch Biochem Biophys. 1988;265:484–495. doi: 10.1016/0003-9861(88)90153-1. [DOI] [PubMed] [Google Scholar]
- 15.Mikelsaar R-H, Scott J E. Glycoconj J. 1994;11:65–71. doi: 10.1007/BF00731145. [DOI] [PubMed] [Google Scholar]