Abstract
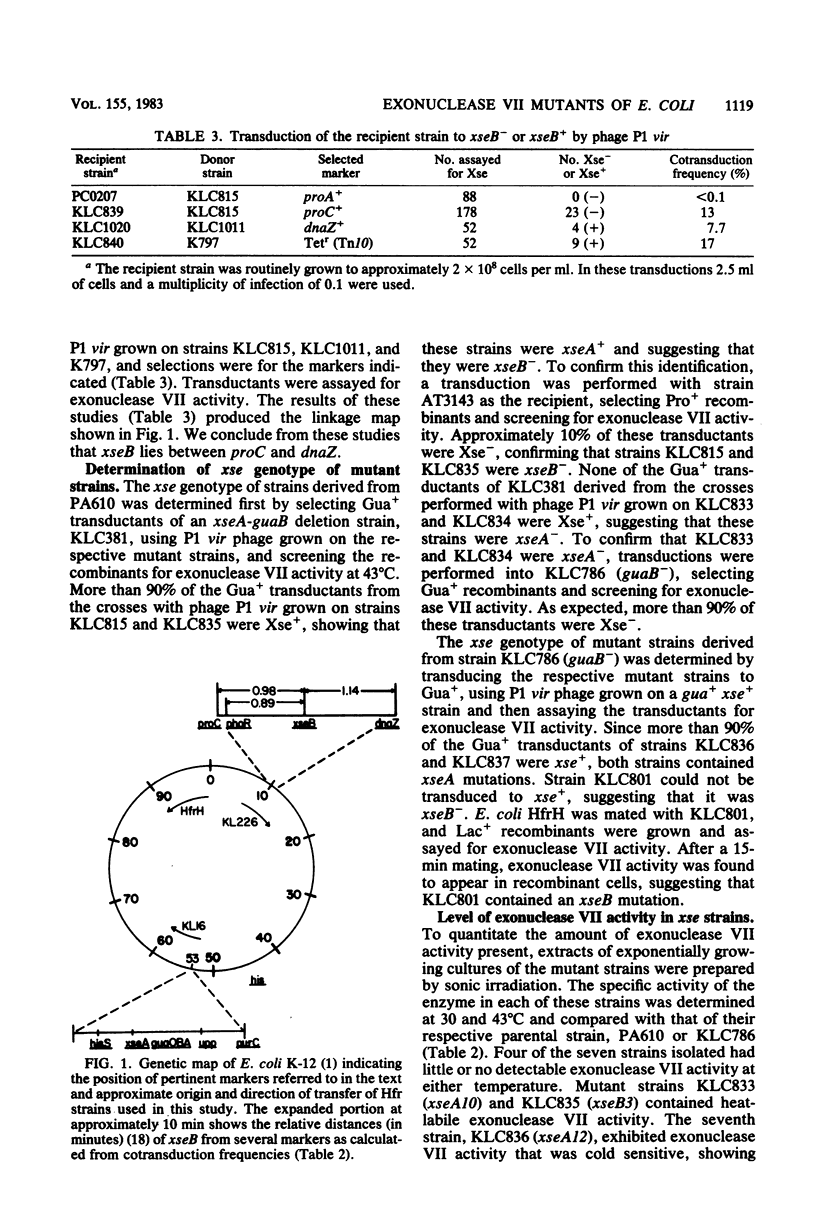
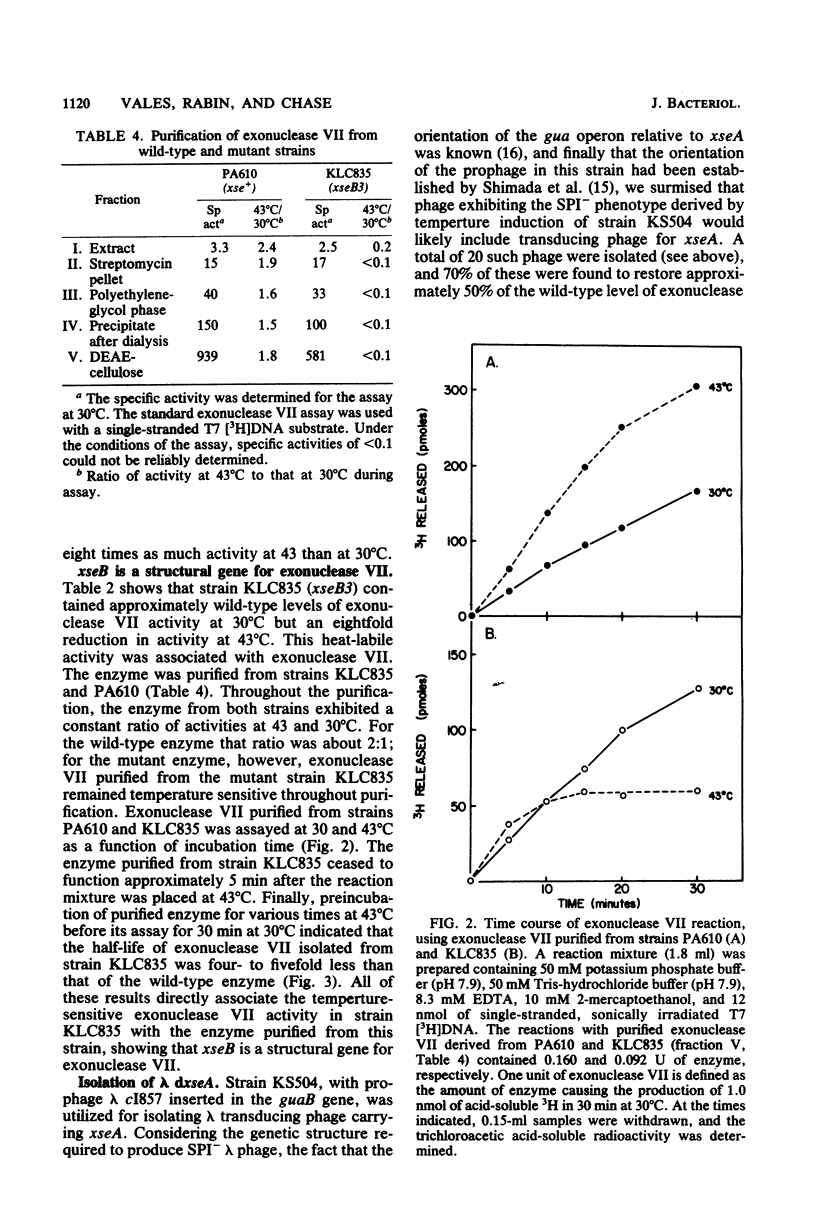
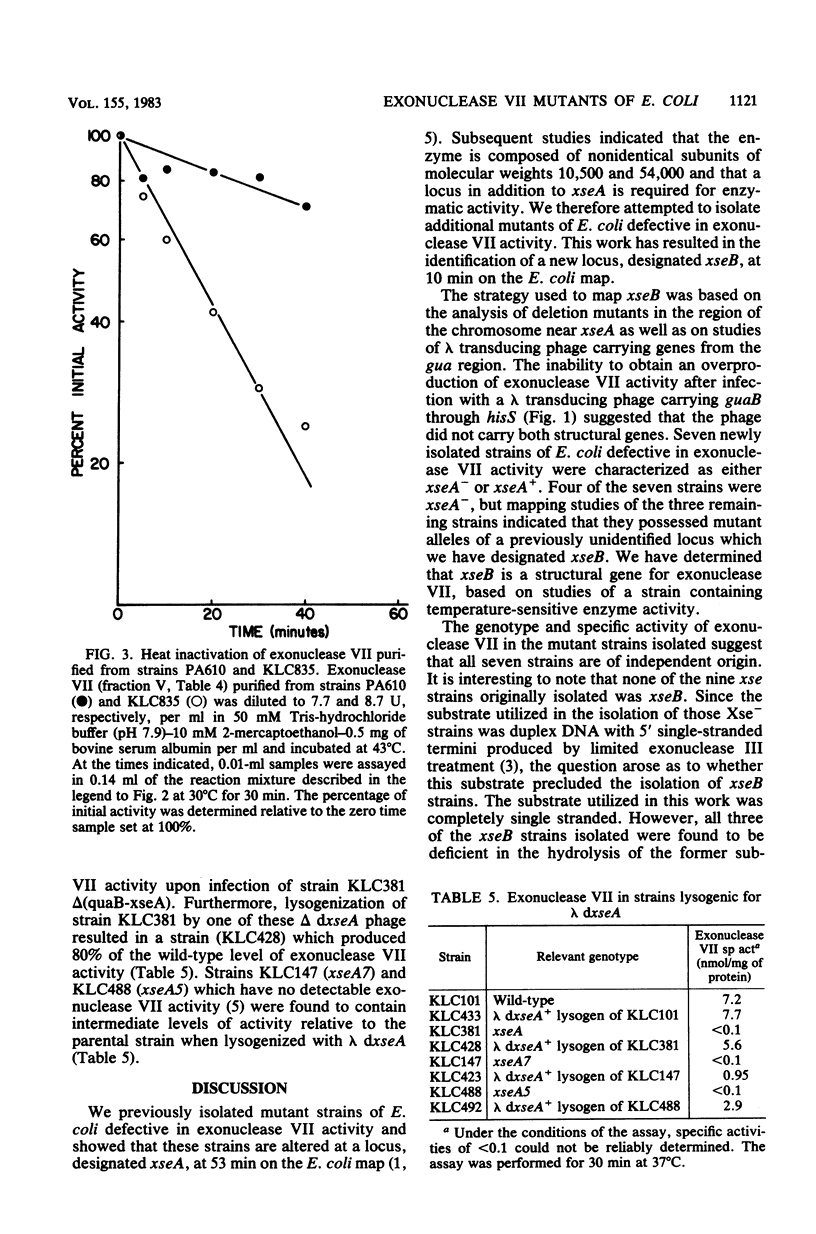
Strains of Escherichia coli containing reduced levels of exonuclease VII activity due to mutations in the xseB gene have been isolated after mutagenesis with N-methyl-N'-nitro-N-nitrosoguanidine. Seven mutants of independent origin deficient in exonuclease VII activity were obtained. Four of these contained defects in xseA, a locus which has been previously identified, and three others contained mutations in a gene distinct from xseA, which we have designated xseB. Genetic mapping studies place the xseB locus between proC and dnaZ. Exonuclease VII purified from KLC835 (xseA+ xseB3) is more heat labile than enzyme purified from the parent strain PA610 (xse+), showing that xseB is a structural gene for exonuclease VII. The isolation of lambda transducing phage carrying xseA is also described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Escherichia coli mutants deficient in exonuclease VII. J Bacteriol. 1977 Feb;129(2):934–947. doi: 10.1128/jb.129.2.934-947.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Excision of DNA segments introduced into cloning vectors by the poly(dA-dT) joining method. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1763–1767. doi: 10.1073/pnas.75.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Straus S. E., Roeder R. G. Transcripts of the adenovirus-associated virus genome: multiple polyadenylated RNAs including a potential primary transcript. J Virol. 1980 Aug;35(2):560–565. doi: 10.1128/jvi.35.2.560-565.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. L., Sunshine M. G. Association of temperate phage P2 with the production of histidine negative segregants by Escherichia coli. Biochem Biophys Res Commun. 1967 Jul 21;28(2):237–243. doi: 10.1016/0006-291x(67)90435-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Oskamp A. A. Regulation of the biosynthesis of guanosine 5'-monophosphate: evidence for one operon. J Mol Biol. 1968 Jul 14;35(1):103–109. doi: 10.1016/s0022-2836(68)80040-3. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979 Jul;92(3):741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Fukumaki Y., Takagi Y. Expression of the guanine operon of Escherichia coli as analyzed by bacteriophage lambda induced mutations. Mol Gen Genet. 1976 Aug 19;147(2):203–208. doi: 10.1007/BF00267572. [DOI] [PubMed] [Google Scholar]
- Vales L. D., Chase J. W., Murphy J. B. Orientation of the guanine operon of Escherichia coli K-12 by utilizing strains containing guaB-xse and guaB-upp deletions. J Bacteriol. 1979 Jul;139(1):320–322. doi: 10.1128/jb.139.1.320-322.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales L. D., Rabin B. A., Chase J. W. Subunit structure of Escherichia coli exonuclease VII. J Biol Chem. 1982 Aug 10;257(15):8799–8805. [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]



