Abstract
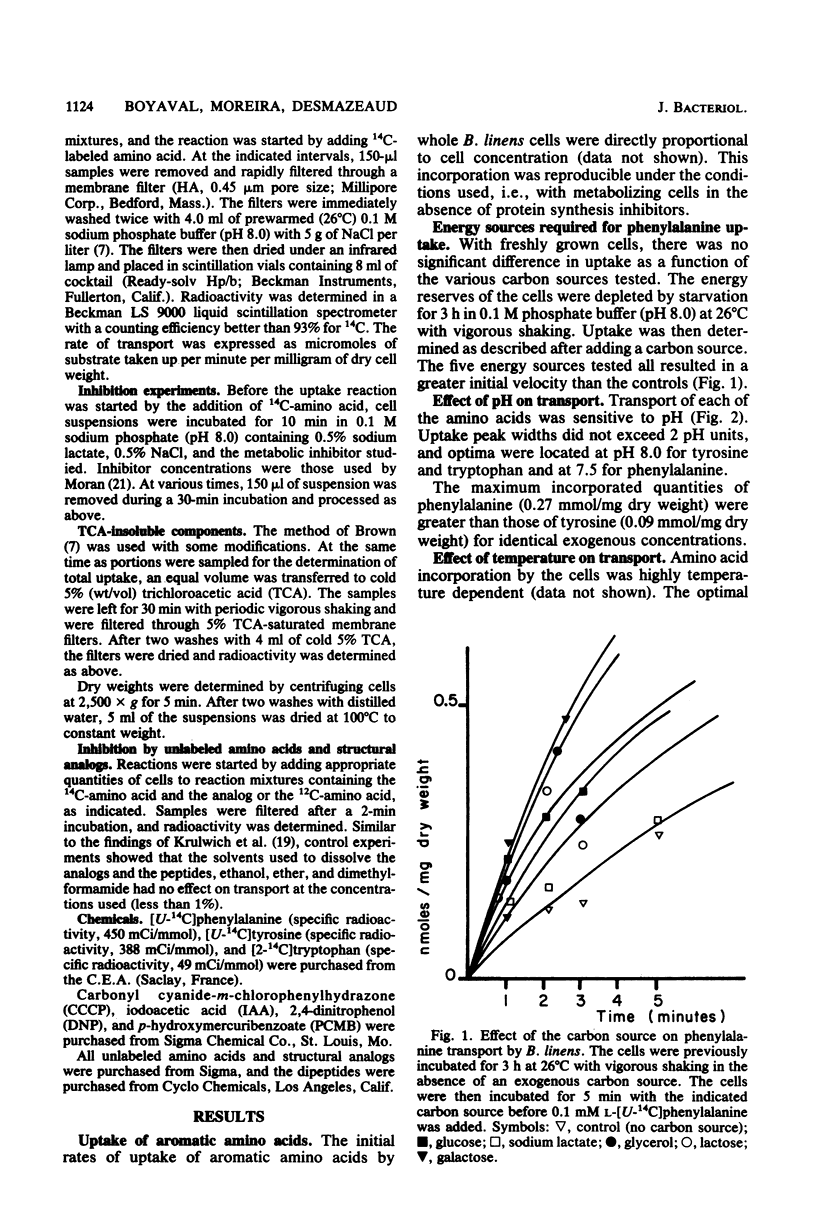
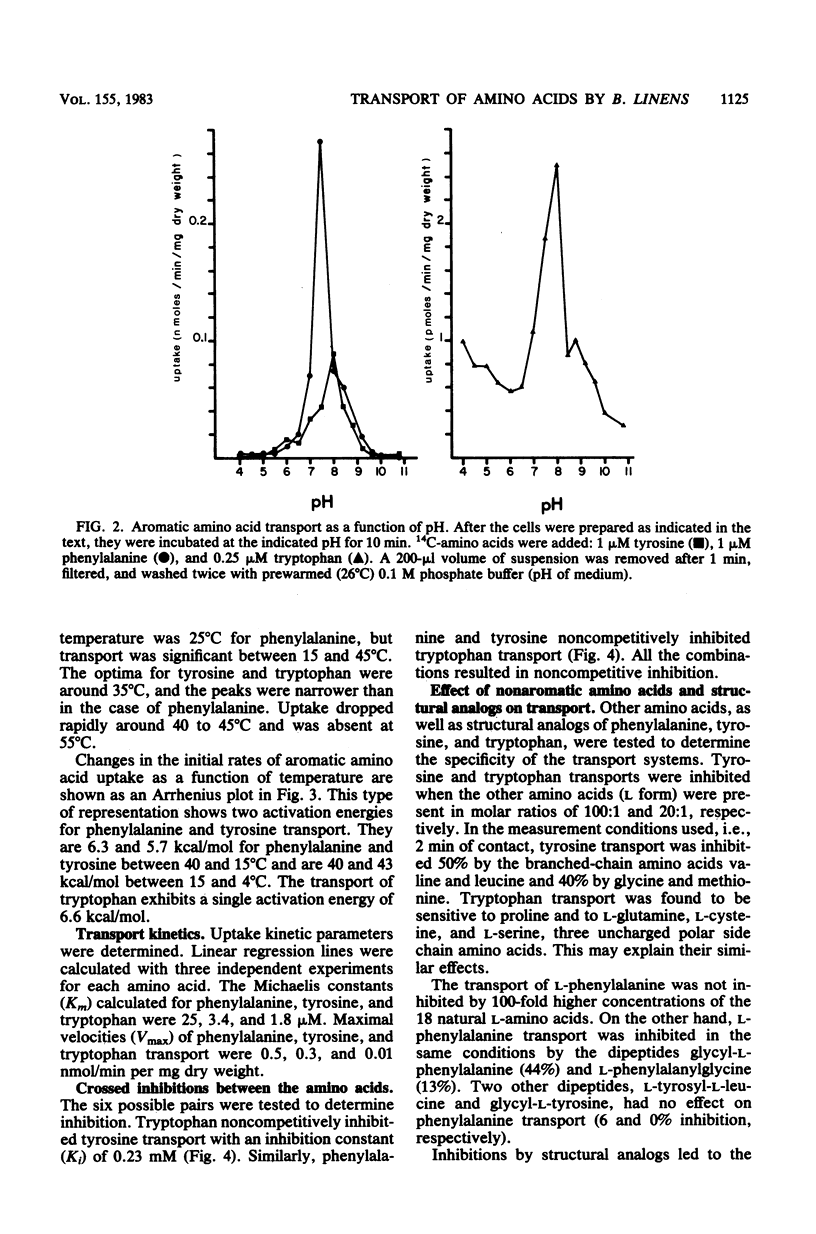
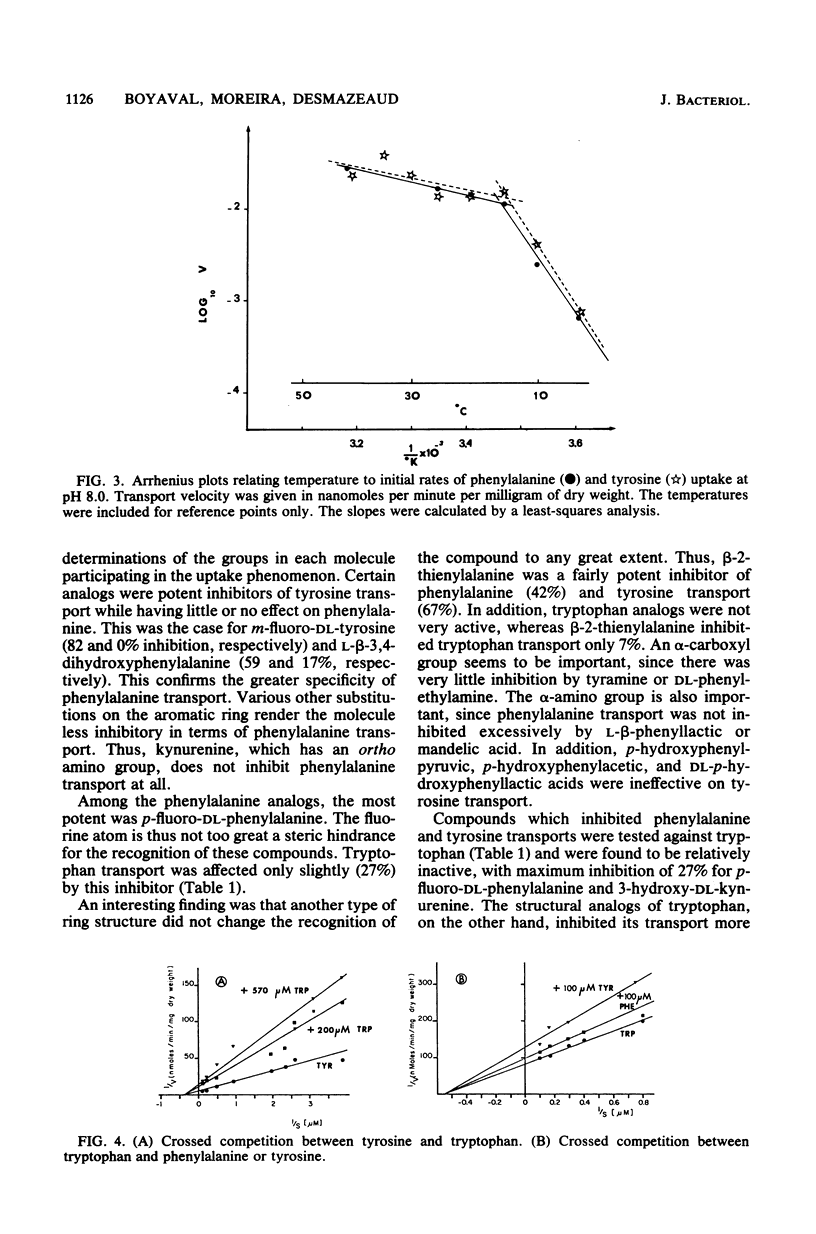
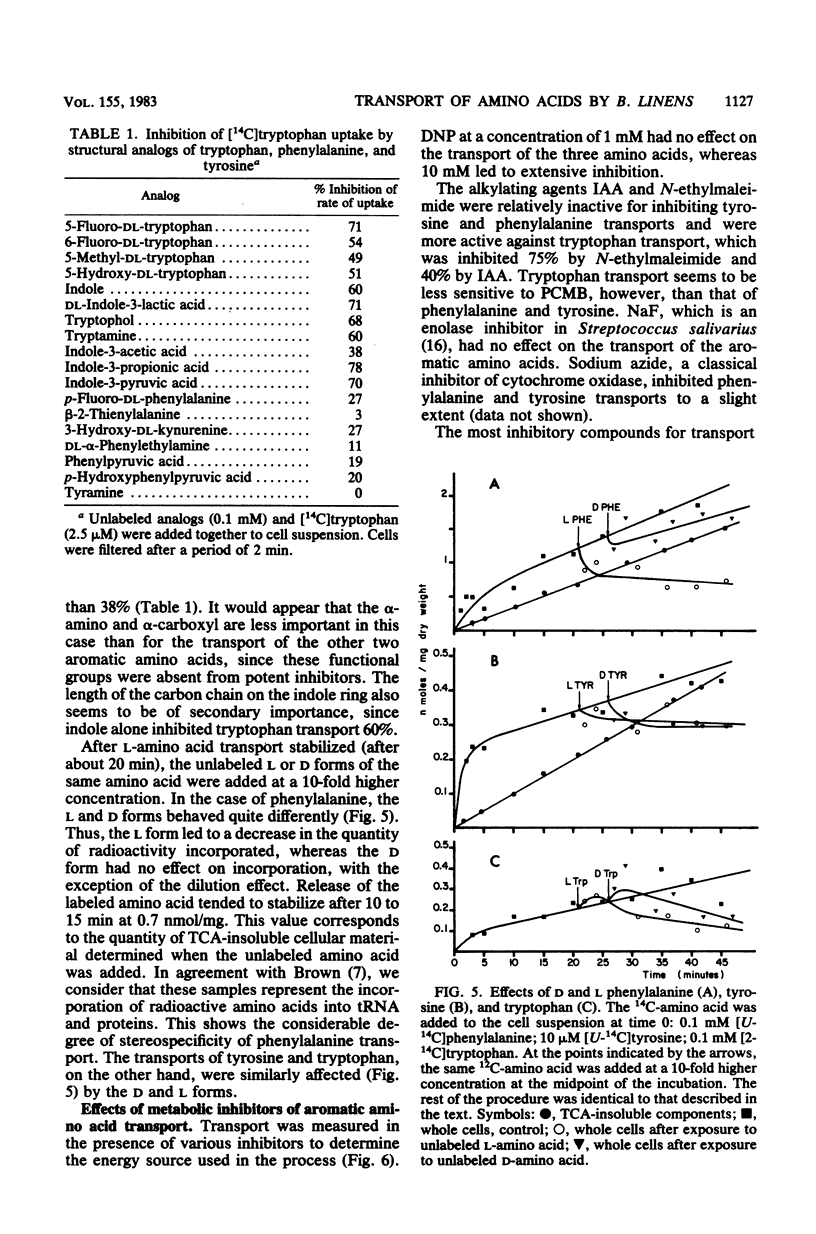
Whole metabolizing Brevibacterium linens cells were used to study the transport of aromatic amino acids. Kinetic results followed the Michaelis-Menten equation with apparent Km values for phenylalanine, tyrosine, and tryptophan of 24, 3.5, and 1.8 microM. Transport of these amino acids was optimum at pH 7.5 and 25 degrees C for phenylalanine and pH 8.0 and 35 degrees C for tyrosine and tryptophan. Crossed inhibitions were all noncompetitive. The only marked stereospecificity was for the L form of phenylalanine. Transport was almost totally inhibited by carbonyl cyanide-m-chlorophenylhydrazone. Iodoacetate and N-ethylmaleimide were much more inhibitory for tryptophan transport than for transport of the other two aromatic amino acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouknight R. R., Sadoff H. L. Transport of D- and L-tryptophan in Bacillus megaterium by an inducible permease. J Bacteriol. 1975 Jan;121(1):65–69. doi: 10.1128/jb.121.1.65-69.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Glover G. I., Nelson S. O., Jensen R. A. Specificity of the tyrosine-phenylalanine transport system in Bacillus subtilis. J Bacteriol. 1973 Aug;115(2):673–681. doi: 10.1128/jb.115.2.673-681.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F., Bramhall J. The origin of the break in Arrhenius plots of membrane processes. Biochim Biophys Acta. 1982 Sep 9;690(2):310–313. doi: 10.1016/0005-2736(82)90337-6. [DOI] [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Isolation of amino acid transport-negative mutants of Pseudomonas aeruginosa and cells with repressed transport activity. J Bacteriol. 1969 Apr;98(1):116–123. doi: 10.1128/jb.98.1.116-123.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Transport of aromatic amino acids by Pseudomonas aeruginosa. J Bacteriol. 1971 Mar;105(3):1039–1046. doi: 10.1128/jb.105.3.1039-1046.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Blanco R., McBride P. A. Amino acid transport in whole cells and membrane vesicles of Arthrobacter pyridinolis. Arch Biochem Biophys. 1977 Jan 15;178(1):108–117. doi: 10.1016/0003-9861(77)90175-8. [DOI] [PubMed] [Google Scholar]
- LUBIN M., KESSEL D. H., BUDREAU A., GROSS J. D. The isolation of bacterial mutants defective in amino acid transport. Biochim Biophys Acta. 1960 Aug 26;42:535–538. doi: 10.1016/0006-3002(60)90836-2. [DOI] [PubMed] [Google Scholar]
- Moran J. W. Branched-chain amino acid transport in Streptococcus agalactiae. Appl Environ Microbiol. 1980 Jul;40(1):25–31. doi: 10.1128/aem.40.1.25-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Rosenfeld H., Feigelson P. Product induction in Pseudomonas acidovorans of a permease system which transports L-tryptophan. J Bacteriol. 1969 Feb;97(2):705–714. doi: 10.1128/jb.97.2.705-714.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M. E., Law B. A., Phillips B. A., Pitcher D. G. Methanethiol production by coryneform bacteria: strains from dairy and human skin sources and Brevibacterium linens. J Gen Microbiol. 1977 Aug;101(2):345–349. doi: 10.1099/00221287-101-2-345. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Montie T. C. Aromatic amino acid transport in Yersinia pestis. J Bacteriol. 1975 Jun;122(3):1045–1052. doi: 10.1128/jb.122.3.1045-1052.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Montie T. C. Separation of phenylalanine transport events by using selective inhibitors, and identification of a specific uncoupler activity in Yersinia pestis. J Bacteriol. 1975 Jun;122(3):1053–1061. doi: 10.1128/jb.122.3.1053-1061.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Dimock K., Martin W. G., Schneider H. Differences in coupling of energy to glycine and phenylalanine transport in aerobically grown Escherichia coli. J Bacteriol. 1975 Sep;123(3):828–836. doi: 10.1128/jb.123.3.828-836.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp M. J., Halsall D. M., Pittard A. J. Isolation and characterization of an Escherichia coli K-12 mutant defective in tyrosine- and phenylalanine-specific transport systems. J Bacteriol. 1980 Jul;143(1):1–7. doi: 10.1128/jb.143.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinbarger L., Jr Mutations in Neurospora crassa which affect multiple amino acid transport systems. Biochim Biophys Acta. 1976 Jul 15;436(4):774–788. doi: 10.1016/0005-2736(76)90405-3. [DOI] [PubMed] [Google Scholar]



