Abstract
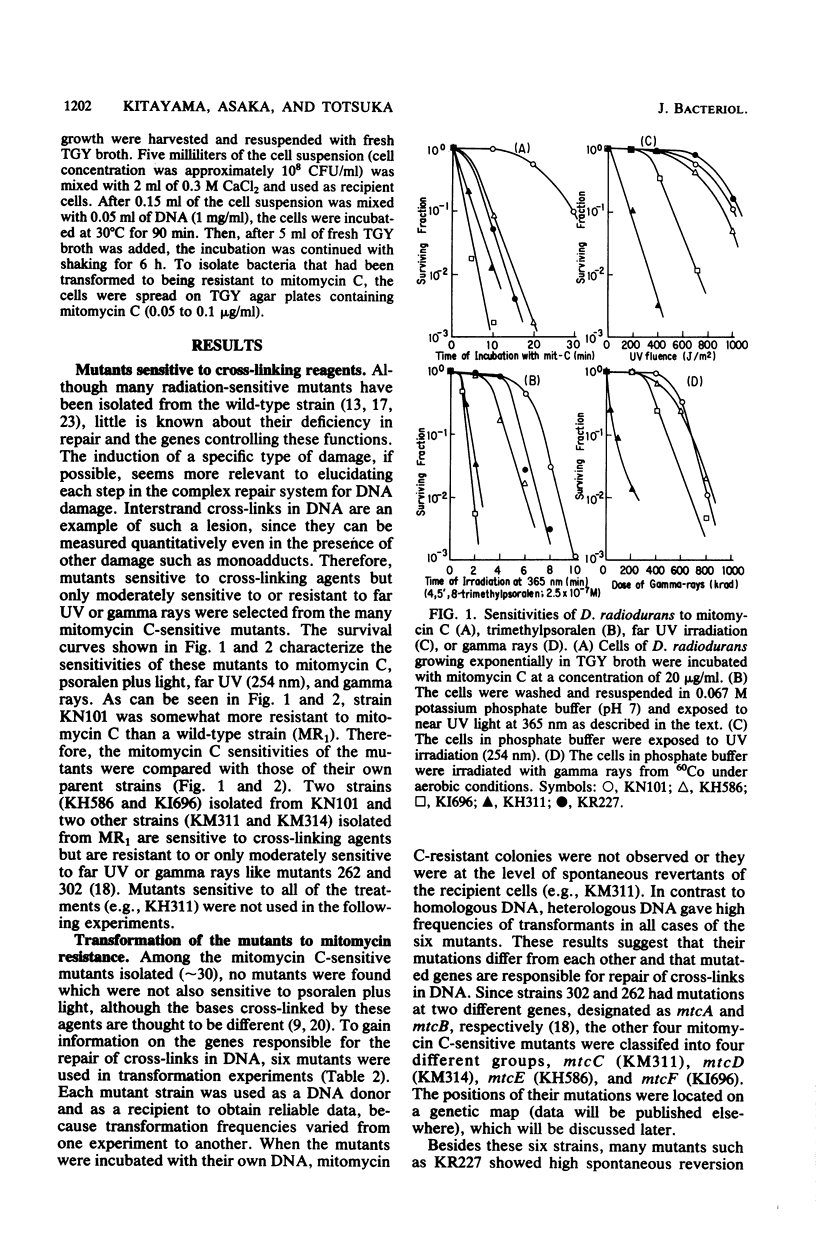
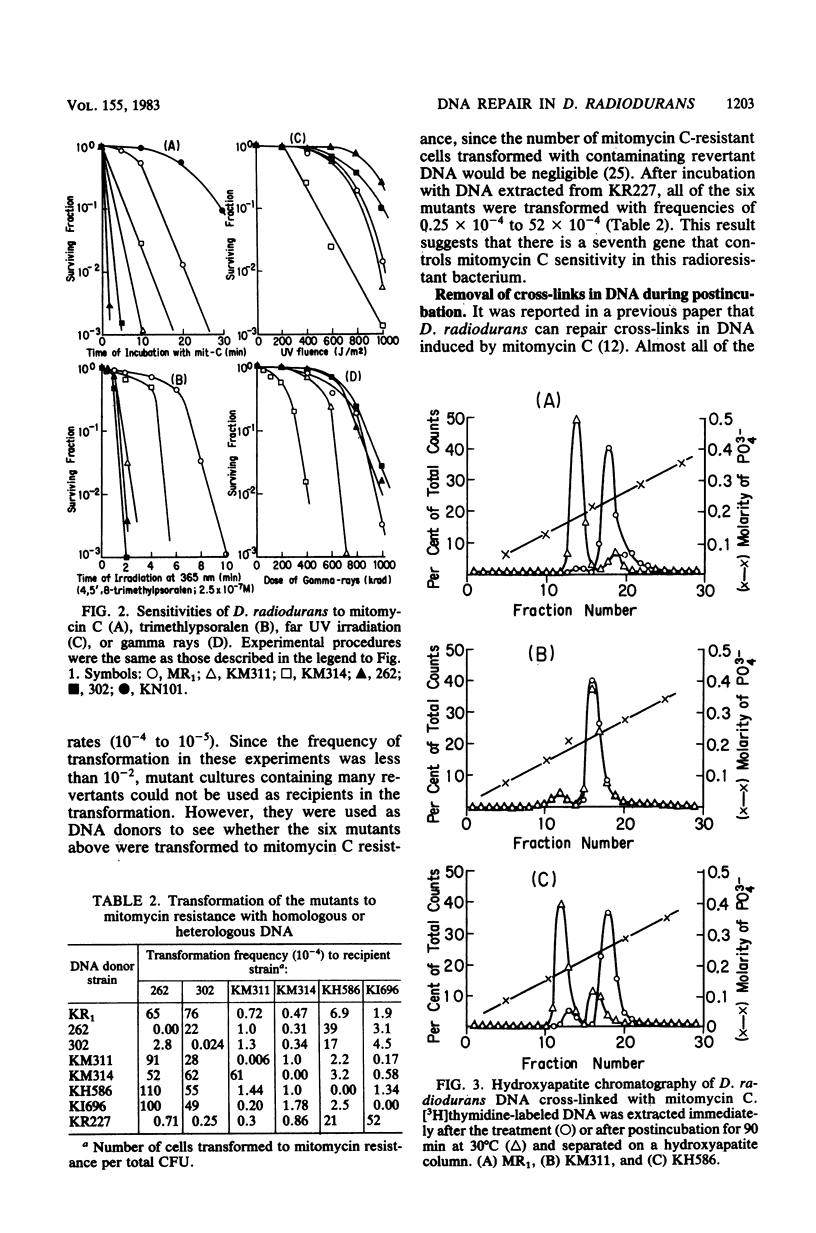
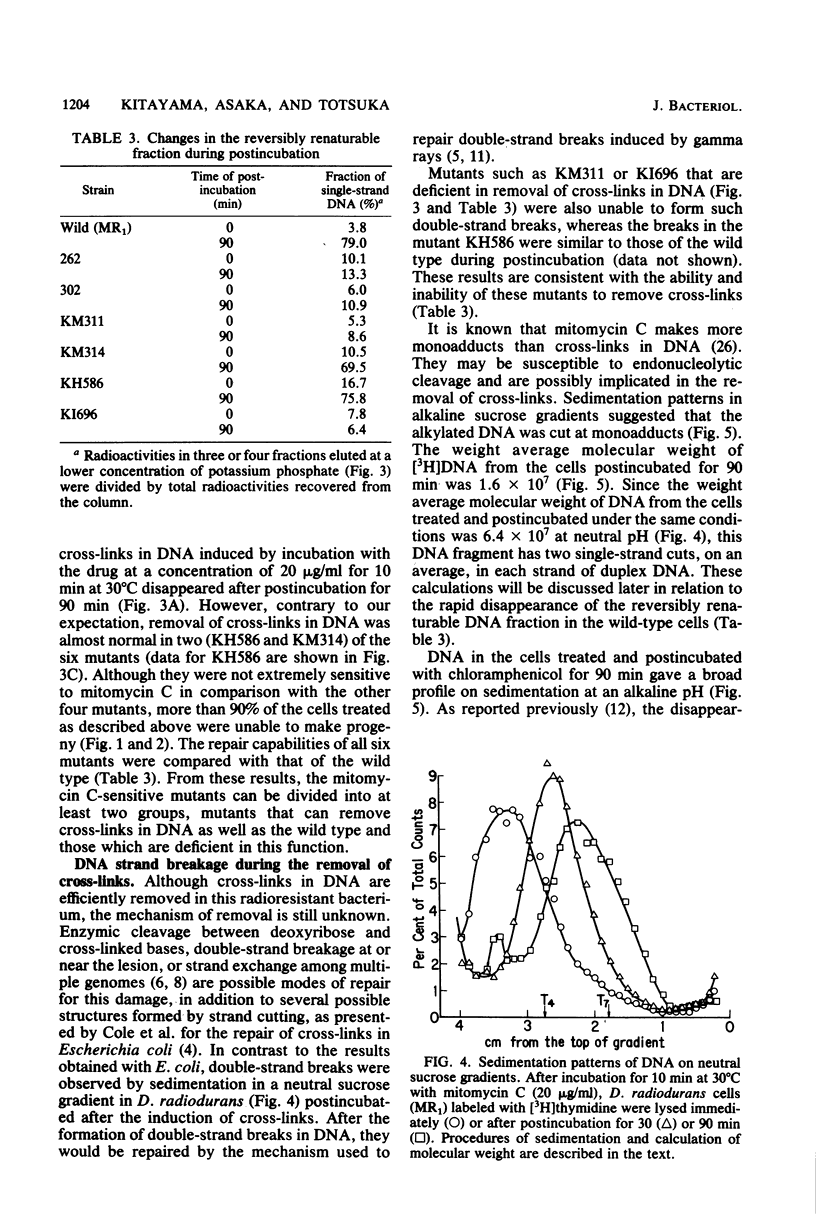
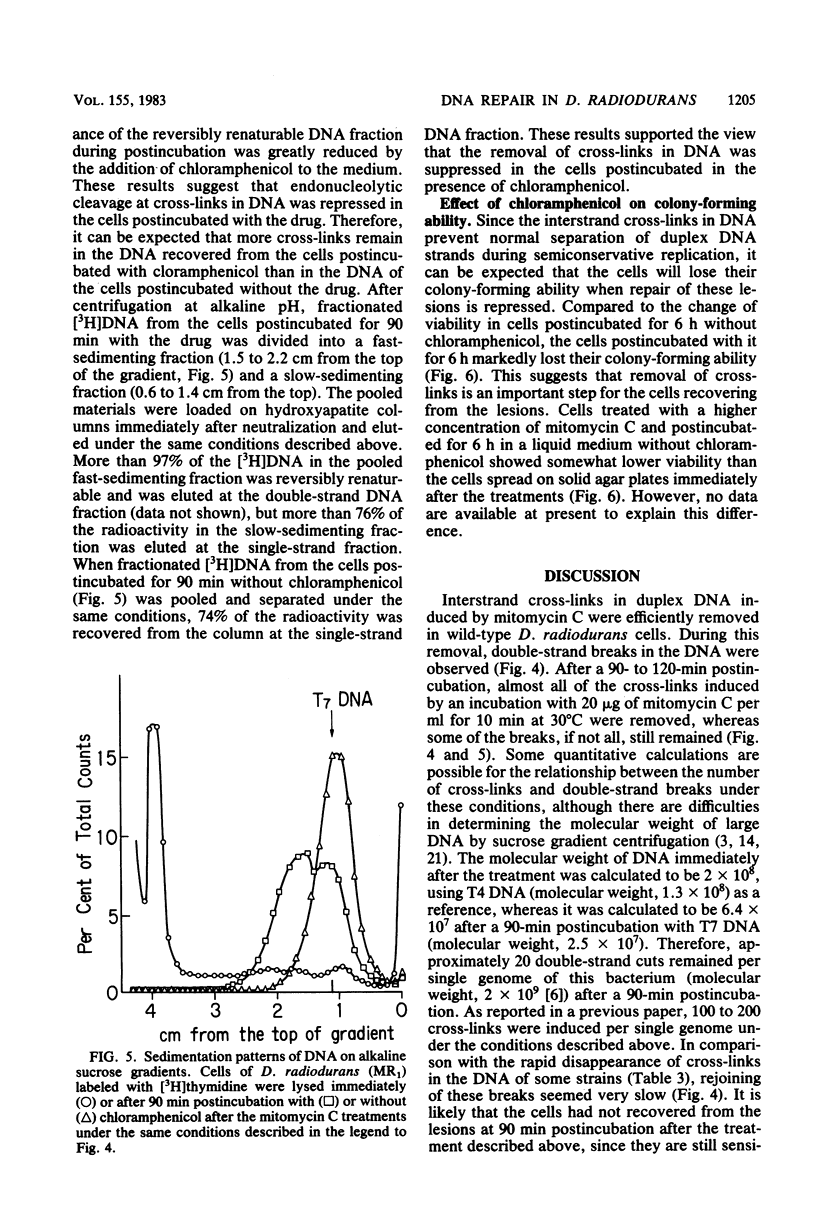
Mitomycin C-sensitive mutants of Deinococcus radiodurans were isolated which were either resistant to or only moderately sensitive to far UV (254 nm) or gamma rays. They were also sensitive to irradiation at 365 nm in the presence of 4,5',8-trimethylpsoralen. They were classified into seven complementary groups (mtcA through mtcG) by transformation experiments. Interstrand cross-links in the DNA duplex induced by mitomycin C were removed in the cells of two mutants (mtcD and mtcE) as in the wild type, whereas the other mutants were deficient in this repair. After a sublethal dosage of mitomycin C, single- and double-stranded cuts of cross-linked DNA were observed in the wild-type cells during postincubation. This removal of cross-links in DNA seems to be indispensable for the cells since their colony-forming ability was markedly reduced if they were postincubated under an inhibitory condition for repair of these lesions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chia D., Schumaker V. N. A rotor speed dependent crossover in sedimentation velocities of DNA's of different sizes. Biochem Biophys Res Commun. 1974 Jan;56(1):241–246. doi: 10.1016/s0006-291x(74)80340-2. [DOI] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Dean C. J., Feldschreiber P., Lett J. T. Repair of x-ray damage to the deoxyribonucleic acid in Micrococcus radiodurans. Nature. 1966 Jan 1;209(5018):49–52. doi: 10.1038/209049a0. [DOI] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation and repair of gamma-ray induced thymine damage in Micrococcus radiodurans. J Mol Biol. 1972 Apr 28;66(1):65–81. doi: 10.1016/s0022-2836(72)80006-8. [DOI] [PubMed] [Google Scholar]
- Harsojo, Kitayama S., Matsuyama A. Genome multiplicity and radiation resistance in Micrococcus radiodurans. J Biochem. 1981 Sep;90(3):877–880. doi: 10.1093/oxfordjournals.jbchem.a133544. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. MITOMYCINS AND PORFIROMYCIN: CHEMICAL MECHANISM OF ACTIVATION AND CROSS-LINKING OF DNA. Science. 1964 Jul 3;145(3627):55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- Kerszman G. Induction of mutation to streptomycin resistance in Micrococcus radiodurans. Mutat Res. 1975 Apr;28(1):9–14. doi: 10.1016/0027-5107(75)90308-5. [DOI] [PubMed] [Google Scholar]
- Kitayama S. Adaptive repair of cross-links in DNA of Micrococcus radiodurans. Biochim Biophys Acta. 1982 Jun 30;697(3):381–384. doi: 10.1016/0167-4781(82)90103-8. [DOI] [PubMed] [Google Scholar]
- Kitayama S., Matsuyama A. Possibility of the repair of double-strand scissions in Micrococcus radiodurans DNA caused by gamma-rays. Biochem Biophys Res Commun. 1968 Nov 8;33(3):418–422. doi: 10.1016/0006-291x(68)90588-3. [DOI] [PubMed] [Google Scholar]
- Levin D., Hutchinson F. Neutral sucrose sedimentation of very large DNA from Bacillus subtilis. I. Effect of random double-strand breaks and centrifuge speed on sedimentation. J Mol Biol. 1973 Apr 15;75(3):455–478. doi: 10.1016/0022-2836(73)90454-3. [DOI] [PubMed] [Google Scholar]
- Masaki T., Tanabe M., Nakamura K., Soejima M. Studies on a new proteolytic enzyme from A chromobacter lyticus M497-1. I. Purification and some enzymatic properties. Biochim Biophys Acta. 1981 Jul 24;660(1):44–50. doi: 10.1016/0005-2744(81)90106-6. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. F. Four mutants of Micrococcus radiodurans defective in the ability to repair DNA damaged by mitomycin-C, two of which have wild-type resistance to ultraviolet radiation. Mol Gen Genet. 1978 Apr 17;160(3):331–337. doi: 10.1007/BF00332977. [DOI] [PubMed] [Google Scholar]
- Moseley B. E. The isolation and some properties of radiation-sensitive mutants of Micrococcus radiodurans. J Gen Microbiol. 1967 Nov;49(2):293–300. doi: 10.1099/00221287-49-2-293. [DOI] [PubMed] [Google Scholar]
- Munson R. J., Neary G. J., Bridges B. A., Preston R. J. The sensitivity of Escherichia coli to ionizing particles of different LETs. Int J Radiat Biol Relat Stud Phys Chem Med. 1967;13(3):205–224. doi: 10.1080/09553006814550141. [DOI] [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schumaker V. N., Zimm B. H. Anomalies in sedimentation. II. Testing the entanglement hypothesis. Biopolymers. 1973 Apr;12(4):869–876. doi: 10.1002/bip.1973.360120415. [DOI] [PubMed] [Google Scholar]
- Sweet D. M., Moseley B. E. Accurate repair of ultraviolet-induced damage in Micrococcus radiodurans. Mutat Res. 1974 Jun;23(3):311–318. doi: 10.1016/0027-5107(74)90104-3. [DOI] [PubMed] [Google Scholar]



