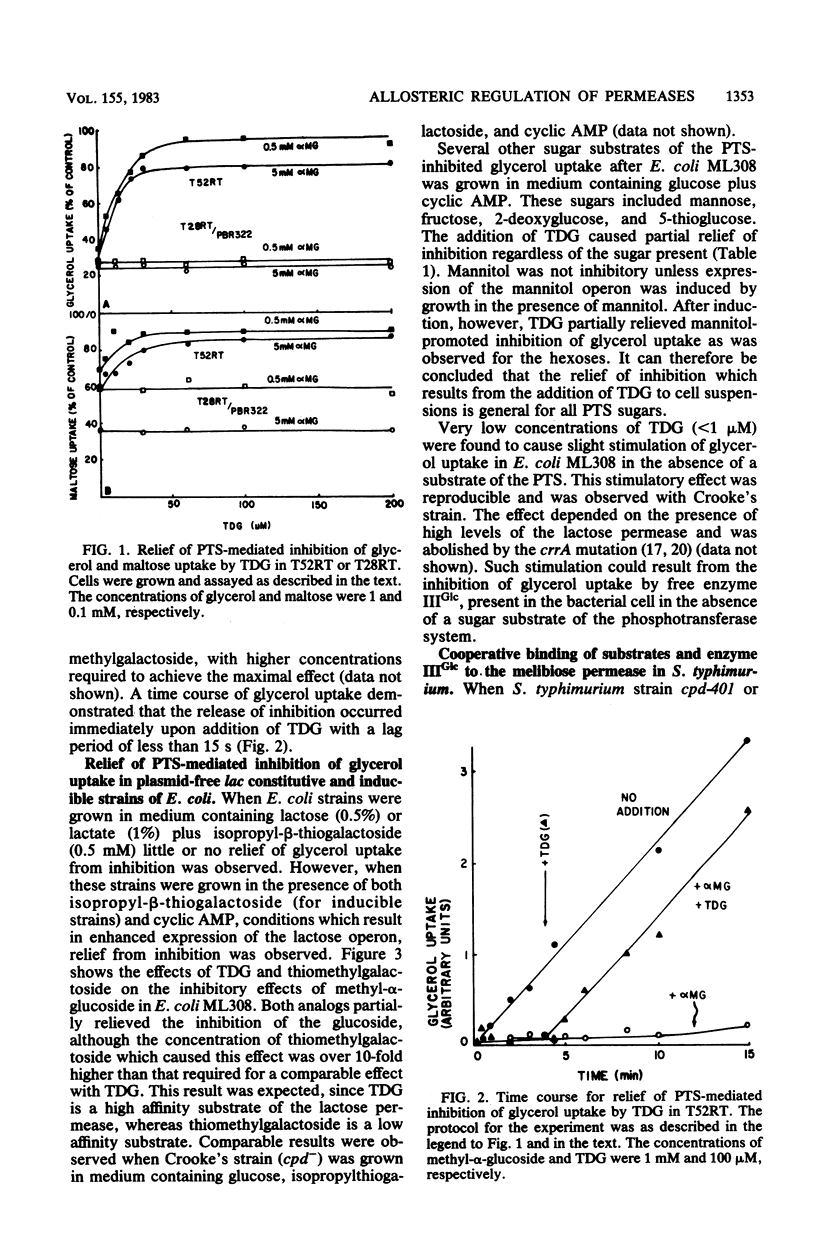
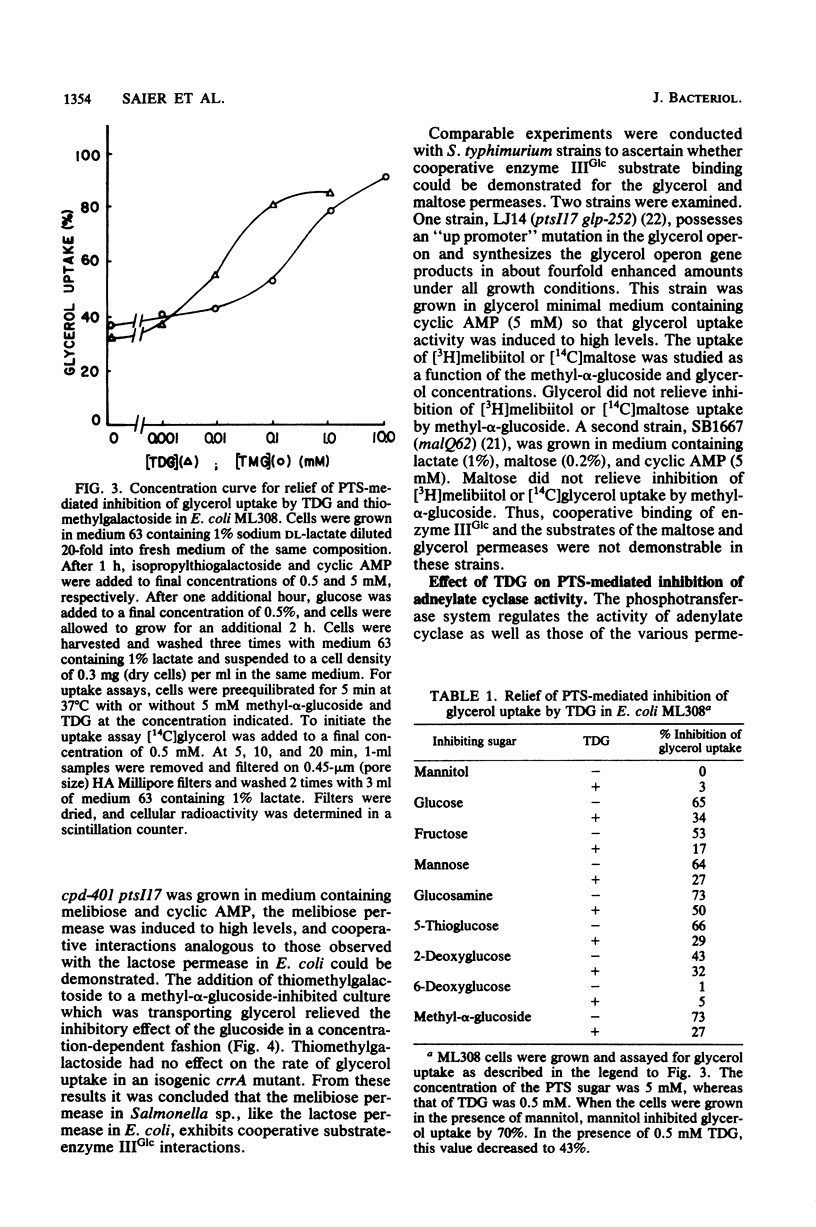
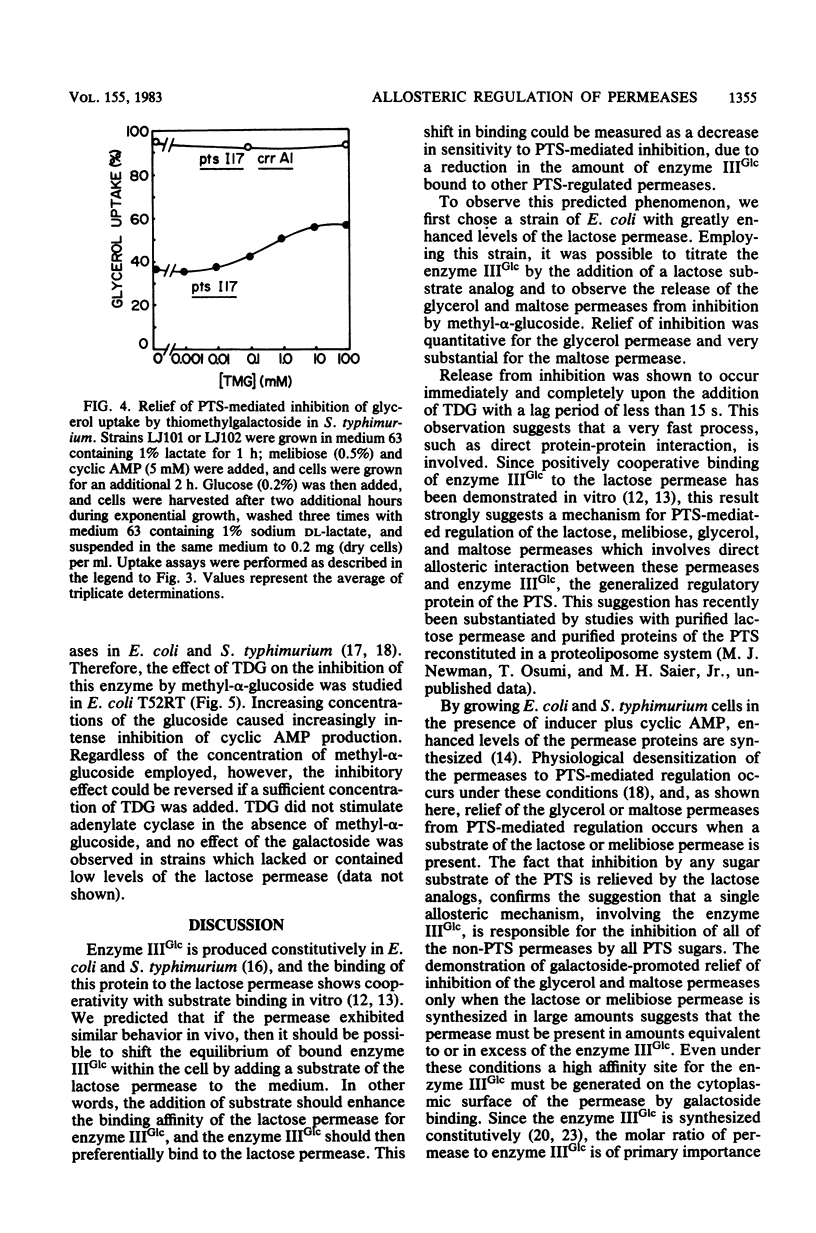
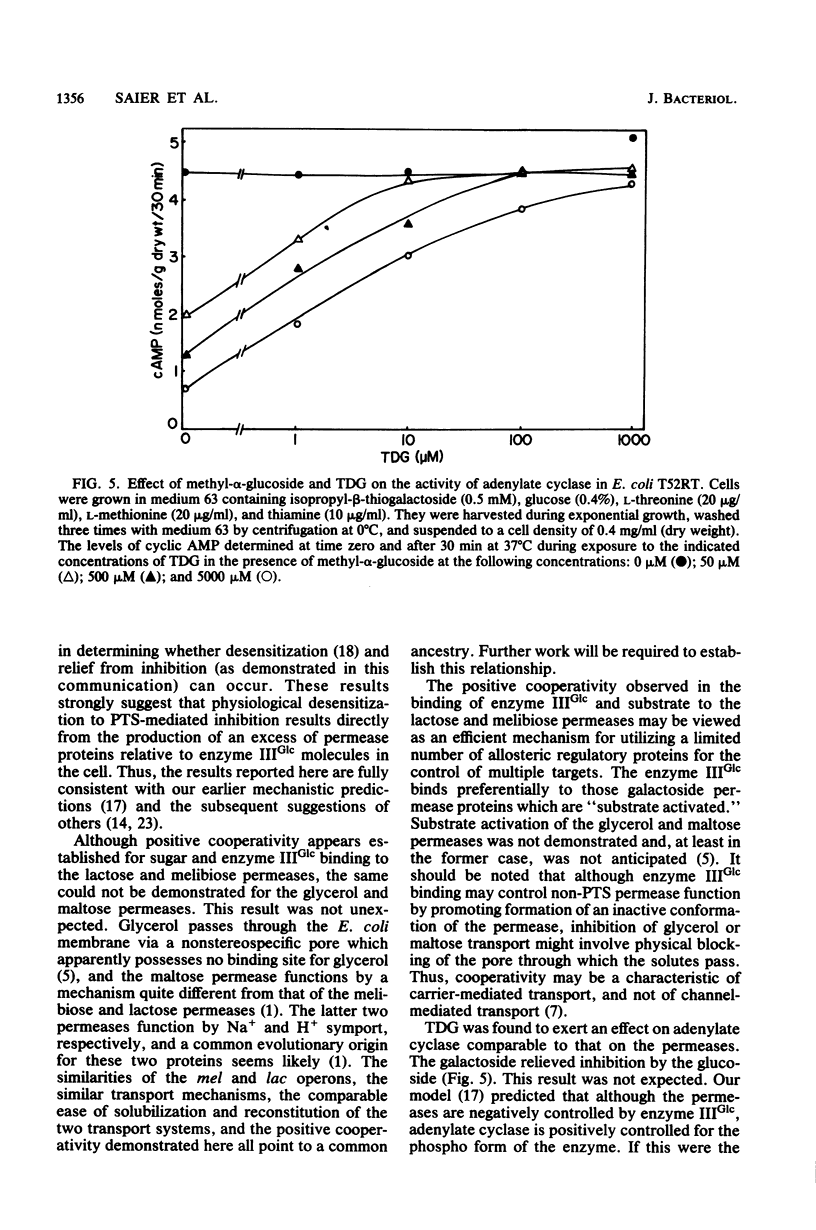
Abstract
An Escherichia coli strain which overproduces the lactose permease was used to investigate the mechanism of allosteric regulation of this permease and those specific for melibiose, glycerol, and maltose by the phosphoenolpyruvate-sugar phosphotransferase system (PTS). Thio-β-digalactoside, a high affinity substrate of the lactose permease, released the glycerol and maltose permeases from inhibition by methyl-α-d-glucoside. Resumption of glycerol uptake occurred immediately upon addition of the galactoside. The effect was not observed in a strain which lacked or contained normal levels of the lactose permease, but growth of wild-type E. coli in the presence of isopropyl-β-thiogalactoside plus cyclic AMP resulted in enhanced synthesis of the lactose permease so that galactosides relieved inhibition of glycerol uptake. Thiodigalactoside also relieved the inhibition of glycerol uptake caused by the presence of other PTS substrates such as fructose, mannitol, glucose, 2-deoxyglucose, and 5-thioglucose. Inhibition of adenylate cyclase activity by methyl-α-glucoside was also relieved by thiodigalactoside in E. coli T52RT provided that the lactose permease protein was induced to high levels. Cooperative binding of sugar and enzyme IIIGlc to the melibiose permease in Salmonella typhimurium was demonstrated, but no cooperativity was noted with the glycerol and maltose permeases. These results are consistent with a mechanism of PTS-mediated regulation of the lactose and melibiose permeases involving a fixed number of allosteric regulatory proteins (enzyme IIIGlc) which may be titrated by the increased number of substrate-activated permease proteins. This work suggests that the cooperativity in the binding of sugar substrate and enzyme IIIGlc to the permease, demonstrated previously in in vitro experiments, has mechanistic significance in vivo. It substantiates the conclusion that PTS-mediated regulation of non-PTS permease activities involves direct allosteric interaction between the permeases and enzyme IIIGlc, the postulated regulatory protein of the PTS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Schmidt M. R., Saier M. H., Jr Regulation of lactose transport by the phosphoenolpyruvate-sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):239–244. doi: 10.1002/jcb.1982.240180211. [DOI] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Jacobson G. R., Saier M. H., Jr Plasmid-directed synthesis of enzymes required for D-mannitol transport and utilization in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7336–7340. doi: 10.1073/pnas.78.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Saffen D. W., Dottin R. P., Roseman S. Molecular cloning of the crr gene and evidence that it is the structural gene for IIIGlc, a phosphocarrier protein of the bacterial phosphotransferase system. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2528–2532. doi: 10.1073/pnas.79.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Scholte B. J., Postma P. W. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):604–615. doi: 10.1128/jb.150.2.604-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Mechanism of regulation of the lactose permease by the phosphotransferase system in Escherichia coli: evidence for protein-protein interaction. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):269–273. [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Keeler D. K., Feucht B. U. Physiological desensitization of carbohydrate permeases and adenylate cyclase to regulation by the phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Involvement of adenosine cyclic 3',5'-phosphate and inducer. J Biol Chem. 1982 Mar 10;257(5):2509–2517. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):257–264. doi: 10.1128/jb.148.1.257-264.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]



