Abstract
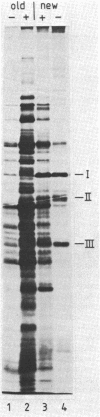
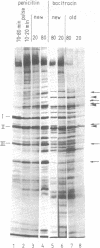
Inhibition of murein biosynthesis in Streptococcus pneumoniae by either penicillin or bacitracin leads to an increase in the amount of protein secreted into the medium. This process was studied in wild-type cells grown under lysis-permissive conditions as well as in an autolysin-deficient mutant. The time course of secretion did not follow cellular lysis but commenced immediately after the addition of the cell wall inhibitor in a manner similar to that described recently for cell wall and membrane components in various tolerant streptococci. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that this increase was not due to the stimulation of release of three protein components which are secreted under normal growth conditions; rather, a complex set of cellular proteins escaped from the antibiotic-treated pneumococci. The proteins released during bacitracin treatment was slightly different from those observed when penicillin was used. Analysis on sucrose gradients indicated that the secreted proteins were membrane bound rather than soluble. Membrane vesicles could indeed be detected by electron microscopy of negative-stained secreted material.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- Calandra G. B., Nugent K. M., Cole R. M. Preparation of protoplasts of group H streptococci (Streptococcus sanguis). Appl Microbiol. 1975 Jan;29(1):90–93. doi: 10.1128/am.29.1.90-93.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fractionation and characterization of the plasma and mesosome membrane of Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):426–440. doi: 10.1128/jb.97.1.426-440.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenawalt J. W., Whiteside T. L. Mesosomes: membranous bacterial organelles. Bacteriol Rev. 1975 Dec;39(4):405–463. doi: 10.1128/br.39.4.405-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
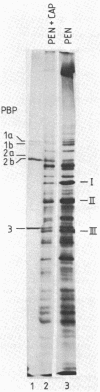
- Gutmann L., Williamson R., Tomasz A. Physiological properties of penicillin-binding proteins in group A streptococci. Antimicrob Agents Chemother. 1981 May;19(5):872–880. doi: 10.1128/aac.19.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Waks S., Tomasz A. Characterization of cell wall polymers secreted into the growth medium of lysis-defective pneumococci during treatment with penicillin and other inhibitors of cell wall synthesis. Antimicrob Agents Chemother. 1978 Feb;13(2):302–311. doi: 10.1128/aac.13.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I. Degradation of phospholipid and release of diglyceride-rich membrane vesicles during protoplast formation in certain gram-positive bacteria. J Bacteriol. 1975 Mar;121(3):1173–1179. doi: 10.1128/jb.121.3.1173-1179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Owen P., Freer J. H. Isolation and properties of mesosomal membrane fractions from Micrococcus lysodeikticus. Biochem J. 1972 Oct;129(4):907–917. doi: 10.1042/bj1290907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin T. J., Theodore T. S., Cole R. M. Electron microscopy during release and purification of mesosomal vesicles and protoplast membranes from Staphylococcus aureus. J Bacteriol. 1971 Sep;107(3):907–917. doi: 10.1128/jb.107.3.907-917.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- Storm D. R., Strominger J. L. Binding of bacitracin to cells and protoplasts of Micrococcus lysodeikticus. J Biol Chem. 1974 Mar 25;249(6):1823–1827. [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]