Abstract
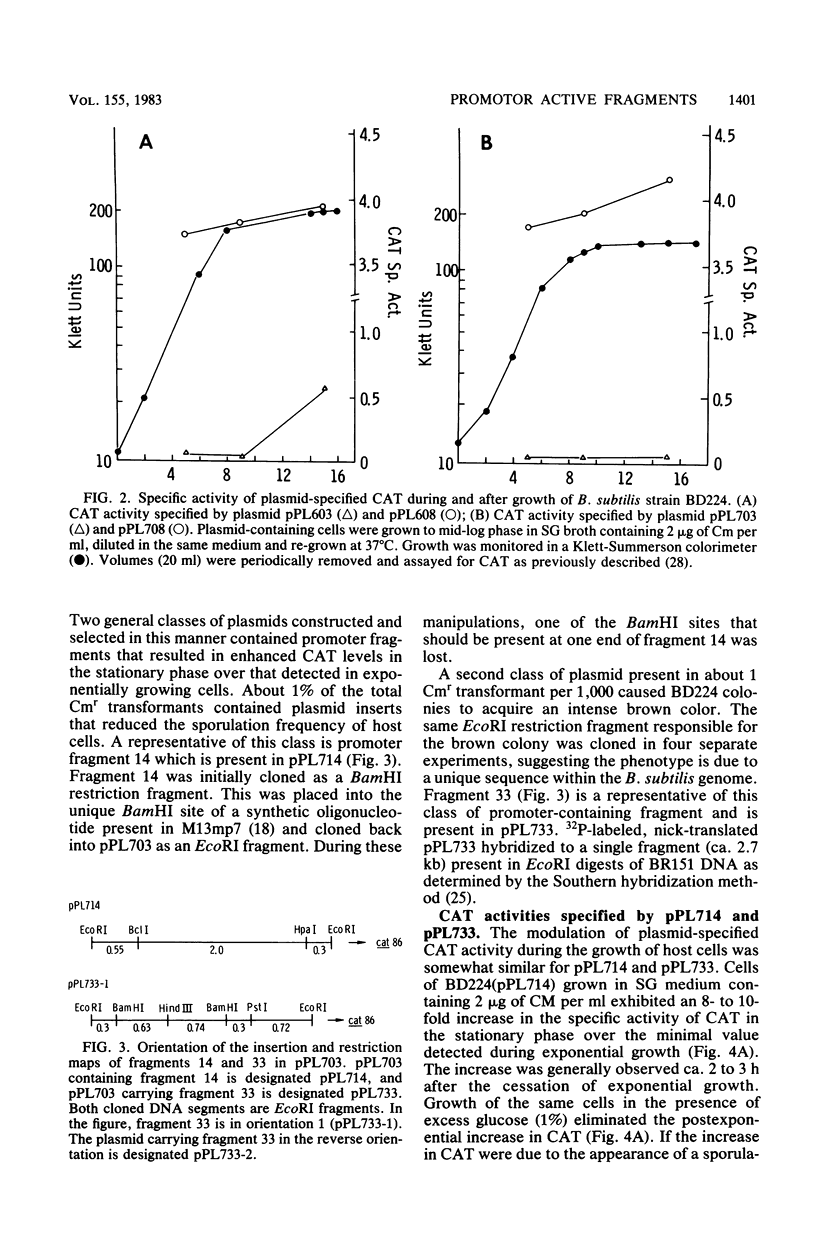
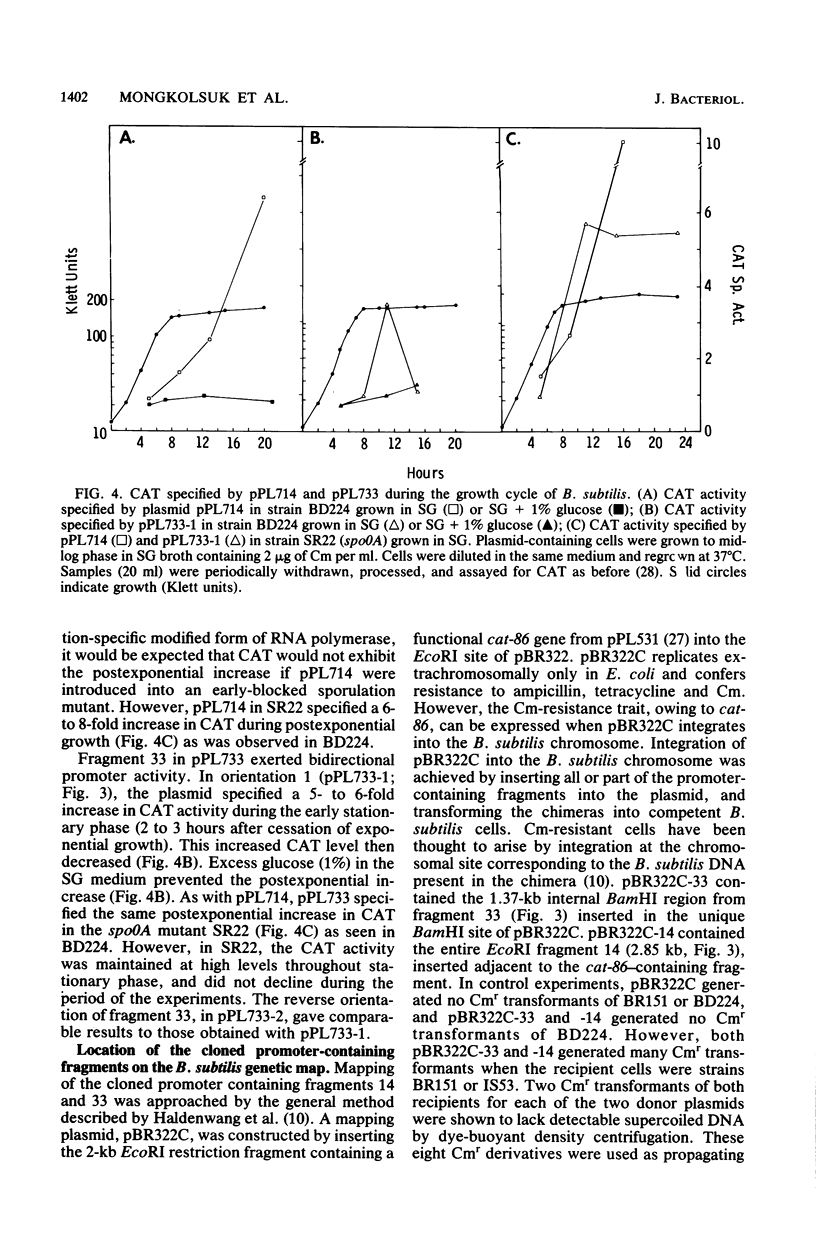
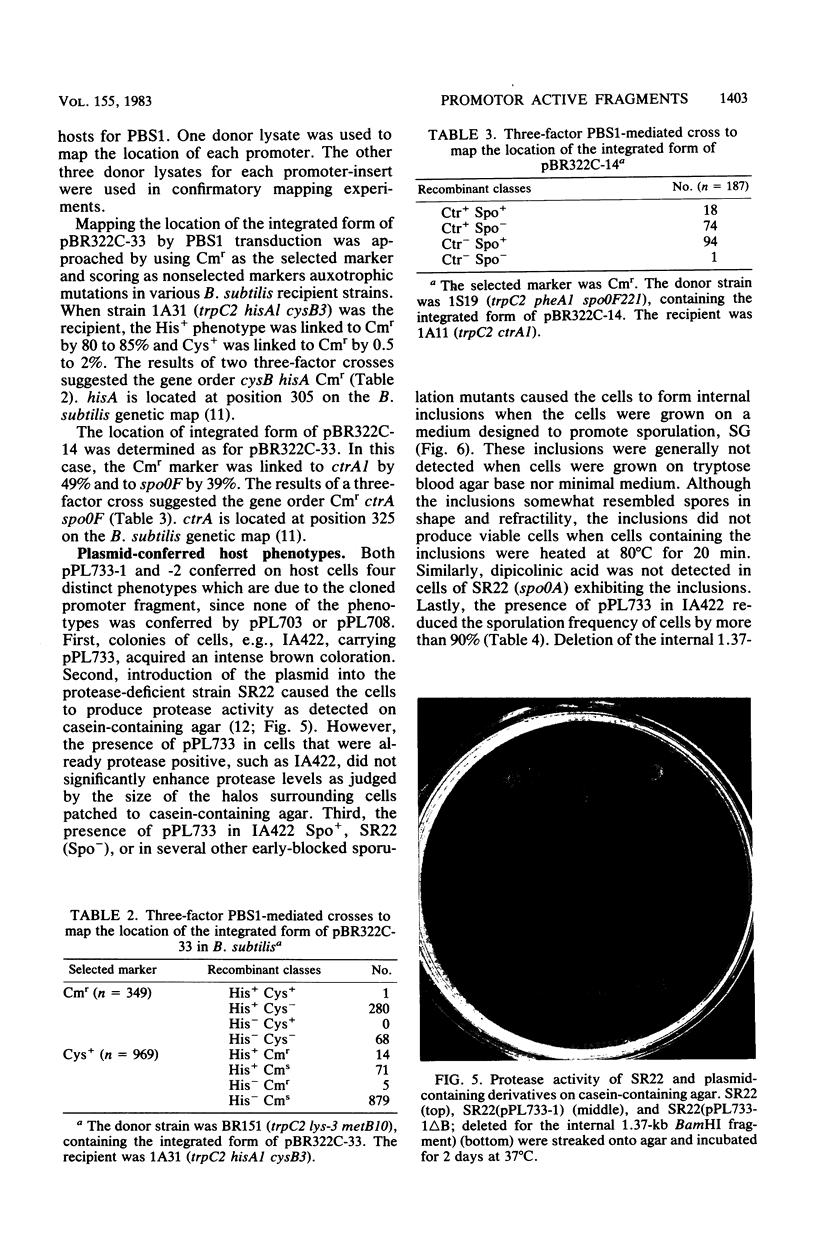
Two restriction fragments of Bacillus subtilis DNA were identified which caused the cat-86 gene present on the promoter cloning plasmid pPL703 to be activated predominantly during postexponential growth of host cells. The postexponential increase was observed in both sporulation-positive strains and in a spoOA mutant of B. subtilis. However, the postexponential increase in the cat-86 gene product, chloramphenicol acetyltransferase, was diminished or not observed when the plasmid-containing cells were grown in the presence of excess glucose. The promoter-containing fragment, designated as 33, was mapped to a site on the B. subtilis chromosome adjacent to hisA. The other fragment, 14, mapped to a site adjacent to ctrA. When present on a high-copy vector, both fragments caused a reduction in the sporulation frequency of host cells. Fragment 33 in high copy number conferred on B. subtilis cells three additional phenotypic changes: brown colony color, intracellular inclusions, and, in a protease-deficient mutant, the production of extracellular protease activity. These activities were observed only in postexponential-phase cultures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramucci M. G., Keggins K. M., Lovett P. S. Bacteriophage PMB12 conversion of the sporulation defect in RNA polymerase mutants of Bacillus subtilis. J Virol. 1977 Oct;24(1):194–200. doi: 10.1128/jvi.24.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Ferrari E., Henner D. J., Hoch J. A. Isolation of Bacillus subtilis genes from a charon 4A library. J Bacteriol. 1981 Apr;146(1):430–432. doi: 10.1128/jb.146.1.430-432.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wiggs J. L., Chamberlin M. J. Nucleotide sequences of two Bacillus subtilis promoters used by Bacillus subtilis sigma-28 RNA polymerase. Nucleic Acids Res. 1981 Nov 25;9(22):5991–6000. doi: 10.1093/nar/9.22.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D. S., Doi R. H., Rodriguez R. L. Expression of Tn9-derived chloramphenicol resistance in Bacillus subtilis. Nature. 1981 Sep 24;293(5830):309–311. doi: 10.1038/293309a0. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higerd T. B. Isolation of acetyl esterase mutants of Bacillus subtilis 168. J Bacteriol. 1977 Feb;129(2):973–977. doi: 10.1128/jb.129.2.973-977.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Talkington C., Pero J. Nucleotide sequence of a promoter recognized by Bacillus subtilis RNA polymerase. Mol Gen Genet. 1980;180(1):57–65. doi: 10.1007/BF00267352. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Keggins K. M. Bacillus subtilis as a host for molecular cloning. Methods Enzymol. 1979;68:342–357. doi: 10.1016/0076-6879(79)68025-4. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., Losick R. Nucleotide sequence of a Bacillus subtilis promoter recognized by Bacillus subtilis RNA polymerase containing sigma 37. Nucleic Acids Res. 1981 Nov 25;9(22):5979–5990. doi: 10.1093/nar/9.22.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Banner C. D., Losick R., Fitz-James P. C. Identification of a new developmental locus in Bacillus subtilis by construction of a deletion mutation in a cloned gene under sporulation control. J Bacteriol. 1981 Oct;148(1):341–351. doi: 10.1128/jb.148.1.341-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Gilman M. Z., Chamberlin M. J. Heterogeneity of RNA polymerase in Bacillus subtilis: evidence for an additional sigma factor in vegetative cells. Proc Natl Acad Sci U S A. 1981 May;78(5):2762–2766. doi: 10.1073/pnas.78.5.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schoner R. G., Duvall E. J., Preis L. H., Lovett P. S. Expression of Escherichia coli trp genes and the mouse dihydrofolate reductase gene cloned in Bacillus subtilis. Gene. 1981 Dec;16(1-3):199–206. doi: 10.1016/0378-1119(81)90076-7. [DOI] [PubMed] [Google Scholar]