Abstract
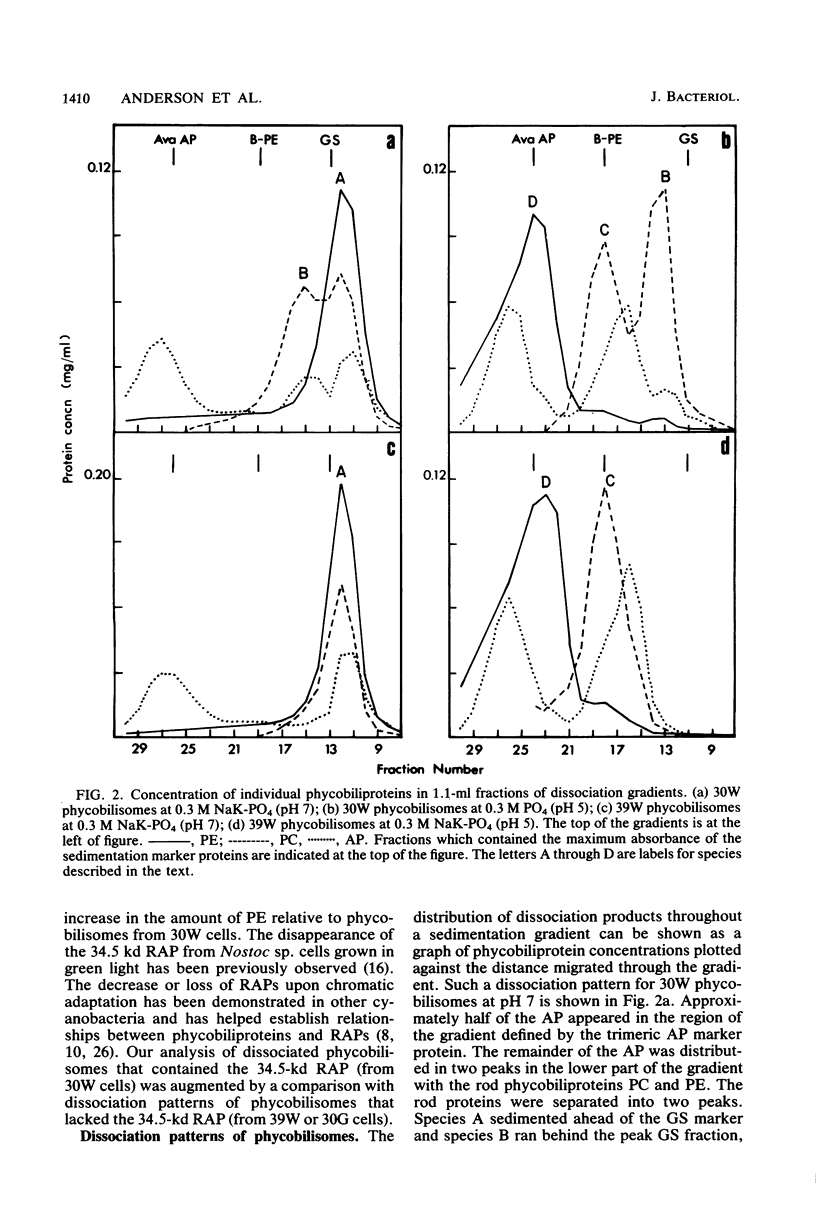
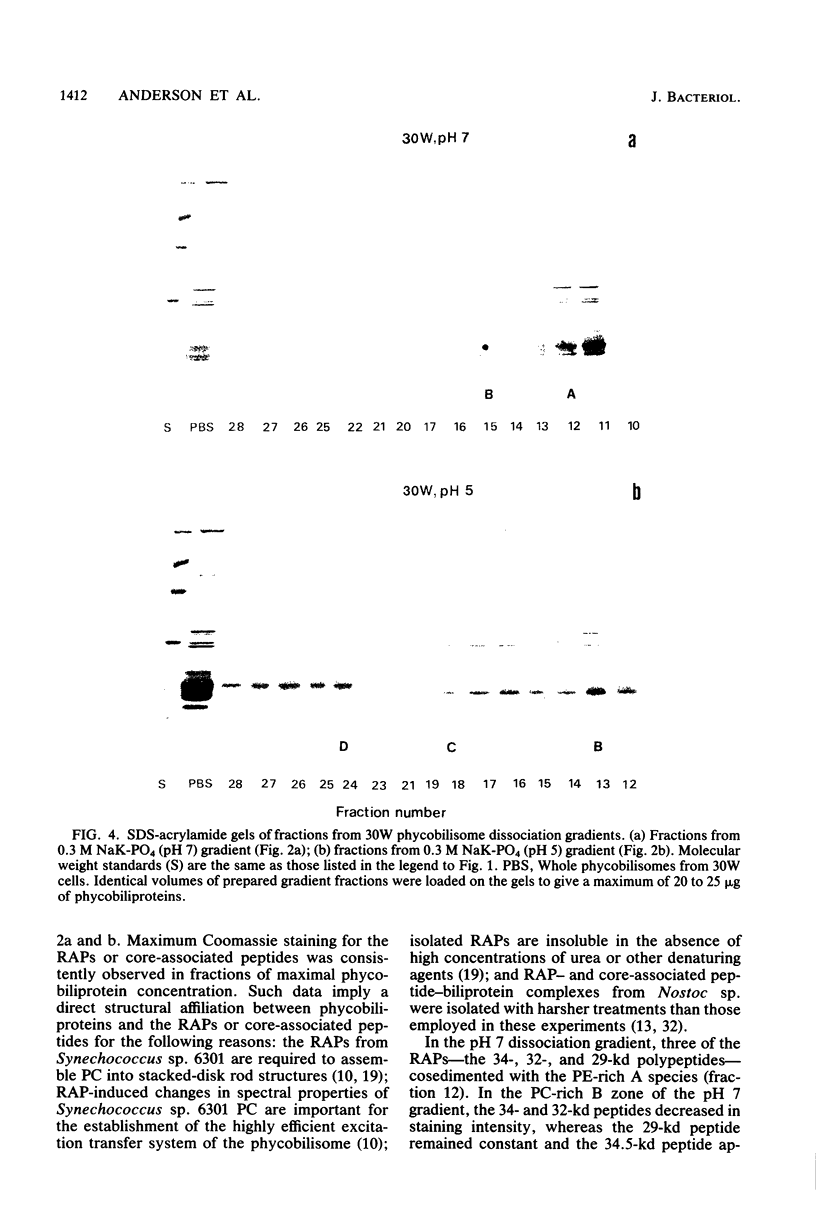
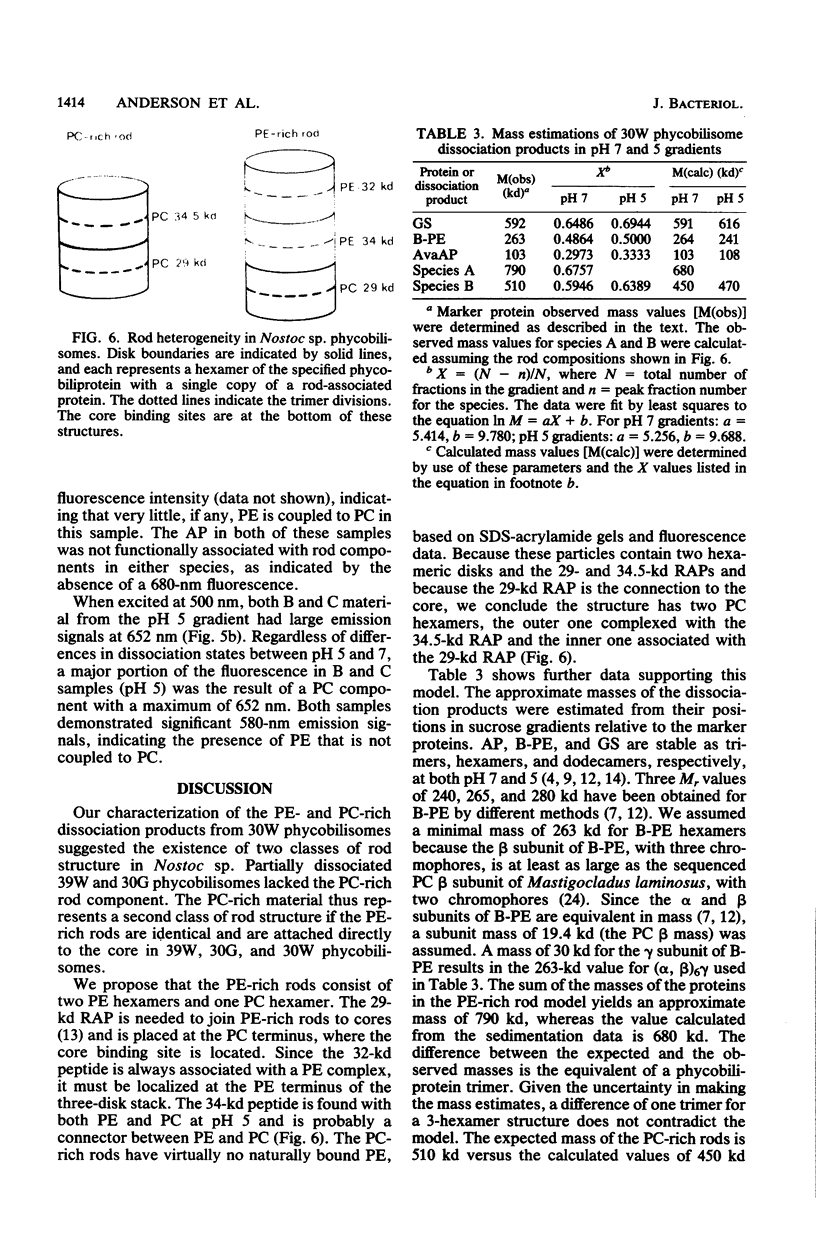
Nostoc sp. strain MAC cyanobacteria were green in color when grown in white light at 30 degrees C and contained phycobilisomes that had phycoerythrin and phycocyanin in a molar ratio of 1:1. Cells grown for 4 to 5 days in green light at 30 degrees C or white light at 39 degrees C turned brown and contained phycoerythrin and phycocyanin in a molar ratio of greater than 2:1. In addition to the change in pigment composition, phycobilisomes from brown cells were missing a 34.5-kilodalton, rod-associated peptide that was present in green cells. The green light-induced changes were typical of the chromatic adaptation response in cyanobacteria, but the induction of a similar response by growth at 39 degrees C was a new observation. Phycobilisomes isolated in 0.65 M phosphate buffer (pH 7) dissociate when the ionic strength or pH is decreased. Analysis of the dissociation products from Nostoc sp. phycobilisomes suggested that the cells contained two types of rod structures: a phycocyanin-rich structure that contained the 34.5-kilodalton peptide and a larger phycoerythrin-rich complex. Brown Nostoc sp. cells that lacked the 34.5-kilodalton peptide also lacked the phycocyanin-rich rod structures in their phycobilisomes. These changes in phycobilisome structure were indistinguishable between cells cultured at 39 degrees C in white light and those cultured at 30 degrees C in green light. A potential role is discussed for rod heterogeneity in the chromatic adaptation response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget P. B., Warner H. R. Identification of P48 and P54 as components of bacteriophage T4 baseplates. J Virol. 1975 Dec;16(6):1669–1677. doi: 10.1128/jvi.16.6.1669-1677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., Cohen-Bazire G. Effects of chromatic illumination on cyanobacterial phycobilisomes. Evidence for the specific induction of a second pair of phycocyanin subunits in Pseudanabaena 7409 grown in red light. Eur J Biochem. 1981 Oct;119(2):415–424. doi: 10.1111/j.1432-1033.1981.tb05624.x. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., Glazer A. N., Eiserling F. A. Characterization and structural properties of the major biliproteins of Anabaena sp. Arch Microbiol. 1976 Oct 11;110(1):61–75. doi: 10.1007/BF00416970. [DOI] [PubMed] [Google Scholar]
- Bryant D. A. The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem. 1981 Oct;119(2):425–429. doi: 10.1111/j.1432-1033.1981.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum: pigment analysis. Biochemistry. 1974 Jul 2;13(14):2960–2966. doi: 10.1021/bi00711a027. [DOI] [PubMed] [Google Scholar]
- Gingrich J. C., Williams R. C., Glazer A. N. Rod substructure in cyanobacterial phycobilisomes: phycoerythrin assembly in synechocystis 6701 phycobilisomes. J Cell Biol. 1982 Oct;95(1):170–178. doi: 10.1083/jcb.95.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Fang S. Formation of hybrid proteins form the and subunits of phycocyanins of unicellular and filamentous blue-green algae. J Biol Chem. 1973 Jan 25;248(2):663–671. [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S. Subunit structure and chromophore composition of rhodophytan phycoerythrins. Porphyridium cruentum B-phycoerythrin and b-phycoerythrin. J Biol Chem. 1977 Jan 10;252(1):32–42. [PubMed] [Google Scholar]
- Glazer A. N. Phycobilisomes: structure and dynamics. Annu Rev Microbiol. 1982;36:173–198. doi: 10.1146/annurev.mi.36.100182.001133. [DOI] [PubMed] [Google Scholar]
- Glick R. E., Zilinskas B. A. Role of the colorless polypeptides in phycobilisome reconstitution from separated phycobiliproteins. Plant Physiol. 1982 May;69(5):991–997. doi: 10.1104/pp.69.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., King J. Genetic control of bacteriophage T4 baseplate morphogenesis. II. Mutants unable to form the central part of the baseplate. J Mol Biol. 1975 Dec 25;99(4):673–694. doi: 10.1016/s0022-2836(75)80179-3. [DOI] [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E., Bryant D. A. Growth and Chromatic Adaptation of Nostoc sp. Strain MAC and the Pigment Mutant R-MAC. Plant Physiol. 1982 Nov;70(5):1549–1553. doi: 10.1104/pp.70.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W., Mörschel E. Biliprotein assemble in the disc-shaped phycobilisomes of Rhodella violacea. On the molecular composition of energy-transfering complexes (tripartite units) forming the periphery of the phycobilisome. Eur J Biochem. 1978 Nov 2;91(1):57–63. doi: 10.1111/j.1432-1033.1978.tb20936.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundell D. J., Williams R. C., Glazer A. N. Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisomes. J Biol Chem. 1981 Apr 10;256(7):3580–3592. [PubMed] [Google Scholar]
- Lundell D. J., Yamanaka G., Glazer A. N. A terminal energy acceptor of the phycobilisome: the 75,000-dalton polypeptide of Synechococcus 6301 phycobilisomes--a new biliprotein. J Cell Biol. 1981 Oct;91(1):315–319. doi: 10.1083/jcb.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlinger T., Gantt E. Phycobilisome Structure of Porphyridium cruentum: POLYPEPTIDE COMPOSITION. Plant Physiol. 1981 Dec;68(6):1375–1379. doi: 10.1104/pp.68.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol. 1977 Apr;130(1):82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gingrich J. C., Glazer A. N. Cyanobacterial phycobilisomes. Particles from Synechocystis 6701 and two pigment mutants. J Cell Biol. 1980 Jun;85(3):558–566. doi: 10.1083/jcb.85.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N. Cyanobacterial phycobilisomes. Role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem. 1982 Apr 10;257(7):3429–3433. [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Phycocyanin assembly in the rod substructures of anabaena variabilis phycobilisomes. J Biol Chem. 1981 Dec 25;256(24):13130–13136. [PubMed] [Google Scholar]
- Zilinskas B. A., Glick R. E. Noncovalent Intermolecular Forces in Phycobilisomes of Porphyridium cruentum. Plant Physiol. 1981 Aug;68(2):447–452. doi: 10.1104/pp.68.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilinskas B. A., Howell D. A. Role of the Colorless Polypeptides in Phycobilisome Assembly in Nostoc sp. Plant Physiol. 1983 Feb;71(2):379–387. doi: 10.1104/pp.71.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilinskas B. A. Isolation and Characterization of the Central Component of the Phycobilisome Core of Nostoc sp. Plant Physiol. 1982 Oct;70(4):1060–1065. doi: 10.1104/pp.70.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]