Abstract
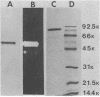
The major inducible trimethylamine oxide reductase was purified from Salmonella typhimurium LT2. The molecular weights of the native enzyme were estimated to be 332,000 by gel filtration and 170,000 by nondenaturing disc gel electrophoresis. In sodium dodecyl sulfate-gel electrophoresis, the enzyme formed a single band of molecular weight 84,000. The isoelectric point was 4.28. Maximum activity was at pH 5.65 and 45 degrees C. Reduced flavin mononucleotide, but not reduced flavin adenine dinucleotide, served as an electron donor. The Km for trimethylamine oxide was 0.89 mM and Vmax was 1,450 U/mg of protein. The enzyme reduced chlorate with a Km of 2.2 mM and a Vmax of 350 U/mg of protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Kim K. E., Chang G. W. Trimethylamine oxide reduction by Salmonella. Can J Microbiol. 1974 Dec;20(12):1745–1748. doi: 10.1139/m74-269. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sagai M., Ishimoto M. An enzyme reducing adenosine 1N-oxide in Escherichia coli, amine N-oxide reductase. J Biochem. 1973 Apr;73(4):843–859. doi: 10.1093/oxfordjournals.jbchem.a130147. [DOI] [PubMed] [Google Scholar]
- Stenberg E., Styrvold O. B., Strøm A. R. Trimethylamine oxide respiration in Proteus sp. strain NTHC153: electron transfer-dependent phosphorylation and L-serine transport. J Bacteriol. 1982 Jan;149(1):22–28. doi: 10.1128/jb.149.1.22-28.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm A. R., Olafsen J. A., Larsen H. Trimethylamine oxide: a terminal electron acceptor in anaerobic respiration of bacteria. J Gen Microbiol. 1979 Jun;112(2):315–320. doi: 10.1099/00221287-112-2-315. [DOI] [PubMed] [Google Scholar]
- Takagi M., Tsuchiya T., Ishimoto M. Proton translocation coupled to trimethylamine N-oxide reduction in anaerobically grown Escherichia coli. J Bacteriol. 1981 Dec;148(3):762–768. doi: 10.1128/jb.148.3.762-768.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]