Abstract
In gram-positive organisms, glyceride-cysteine thioether lipoproteins are frequently associated with secretion. They constitute membrane-bound forms retained by the cell but releasable late in growth phase. Most gram-negative organisms secrete very few proteins to the culture fluid; thioether lipoproteins in such organisms, typified by the enteric bacterium Escherichia coli, are integral outer membrane components for the most part. Unusual among gram-negative organisms, however, are Pseudomonas strains, known for extracellular export of a number of proteins. To examine whether a fundamental difference exists between the processing of lipoproteins in Pseudomonas strains and in nonsecretory gram-negative organisms, we examined the fate in Pseudomonas aeruginosa and E. coli of a cloned gram-positive secretory lipoprotein, Bacillus licheniformis penicillinase. A nonlipoprotein deletion mutant of the same gene was also examined in P. aeruginosa, and its processing was compared with that in E. coli. No important differences were found between P. aeruginosa and E. coli for either the lipoprotein or its deletion mutant. Thus, the contrast in secretory abilities of the two organisms does not appear to result from a difference in their general secretory systems.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatti A. R., DeVoe I. W., Ingram J. M. The release and characterization of some periplasm-located enzymes of Pseudomona aeruginosa. Can J Microbiol. 1976 Oct;22(10):1425–1429. doi: 10.1139/m76-211. [DOI] [PubMed] [Google Scholar]
- Brammar W. J., Muir S., McMorris A. Molecular cloning of the gene for the beta-lactamase of Bacillus licheniformis and its expression in Escherichia coli. Mol Gen Genet. 1980 Apr;178(1):217–224. doi: 10.1007/BF00267232. [DOI] [PubMed] [Google Scholar]
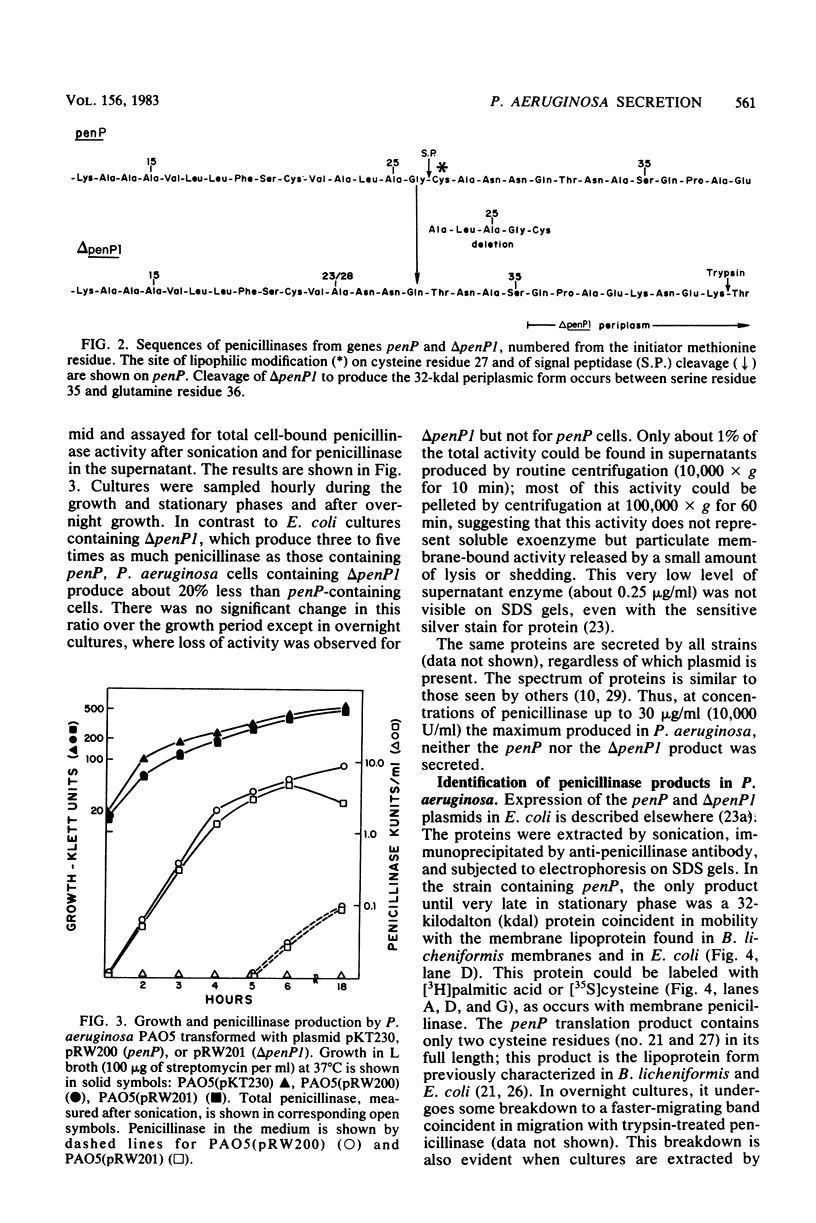
- Chang C. N., Nielsen J. B., Izui K., Blobel G., Lampen J. O. Identification of the signal peptidase cleavage site in Bacillus licheniformis prepenicillinase. J Biol Chem. 1982 Apr 25;257(8):4340–4344. [PubMed] [Google Scholar]
- Coleman K., Dougan G., Arbuthnott J. P. Cloning, and expression in Escherichia coli K-12, of the chromosomal hemolysin (phospholipase C) determinant of Pseudomonas aeruginosa. J Bacteriol. 1983 Feb;153(2):909–915. doi: 10.1128/jb.153.2.909-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESSELMANN M. T., LIU P. V. Lecithinase production by gramnegative bacteria. J Bacteriol. 1961 Jun;81:939–945. doi: 10.1128/jb.81.6.939-945.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro M. L., Greenaway P. J., Broadbent D. A. The expression of biologically active cholera toxin in Escherichia coli. Nucleic Acids Res. 1982 Aug 25;10(16):4883–4890. doi: 10.1093/nar/10.16.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. Phospholipase C regulatory mutation of Pseudomonas aeruginosa that results in constitutive synthesis of several phosphate-repressible proteins. J Bacteriol. 1982 Jun;150(3):1221–1226. doi: 10.1128/jb.150.3.1221-1226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Poole K., Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982 May;150(2):730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Homma J. Y. Roles of exoenzymes and exotoxin in the pathogenicity of Pseudomonas aeruginosa and the development of a new vaccine. Jpn J Exp Med. 1980 Jun;50(3):149–165. [PubMed] [Google Scholar]
- Hou C. I., Gronlund A. F., Campbell J. J. Influence of phosphate starvation on cultures of Pseudomonas aeruginosa. J Bacteriol. 1966 Oct;92(4):851–855. doi: 10.1128/jb.92.4.851-855.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui K., Nielsen J. B., Caulfield M. P., Lampen J. O. Large exopenicillinase, initial extracellular form detected in cultures of Bacillus licheniformis. Biochemistry. 1980 Apr 29;19(9):1882–1886. doi: 10.1021/bi00550a023. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Henning U. Limiting availability of binding sites for dehydrogenases on the cell membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):925–929. doi: 10.1073/pnas.69.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Sarvas M., Brammar W. J., Neugebauer K., Wu H. C. Bacillus licheniformis penicillinase synthesized in Escherichia coli contains covalently linked fatty acid and glyceride. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3506–3510. doi: 10.1073/pnas.78.6.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mézes P. S., Wang W., Yeh E. C., Lampen J. O. Construction of penP delta 1, Bacillus licheniformis 749/C beta-lactamase lacking site for lipoprotein modification. Expression in Escherichia coli and Bacillus subtilis. J Biol Chem. 1983 Sep 25;258(18):11211–11218. [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Glyceride-cysteine lipoproteins and secretion by Gram-positive bacteria. J Bacteriol. 1982 Oct;152(1):315–322. doi: 10.1128/jb.152.1.315-322.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Membrane-bound penicillinases in Gram-positive bacteria. J Biol Chem. 1982 Apr 25;257(8):4490–4495. [PubMed] [Google Scholar]
- Nielsen J. B. Penicillinase secretion in vivo and in vitro. Methods Enzymol. 1983;97:153–158. doi: 10.1016/0076-6879(83)97129-x. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Cryz S. J., Iglewski B. H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980 Jun;142(3):836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Palva I., Pettersson R. F., Kalkkinen N., Lehtovaara P., Sarvas M., Söderlund H., Takkinen K., Käriäinen L. Nucleotide sequence of the promoter and NH2-terminal signal peptide region of the alpha-amylase gene from Bacillus amyloliquefaciens. Gene. 1981 Oct;15(1):43–51. doi: 10.1016/0378-1119(81)90103-7. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair M. I., Morgan A. F. Transformation of Pseudomonas aeruginosa strain PAO with bacteriophage and plasmid DNA. Aust J Biol Sci. 1978 Dec;31(6):679–688. doi: 10.1071/bi9780679. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wolfe P. B., Wu H. C. Prolipoprotein signal peptidase in Escherichia coli is distinct from the M13 procoat protein signal peptidase. J Biol Chem. 1982 Sep 10;257(17):9922–9925. [PubMed] [Google Scholar]