Abstract
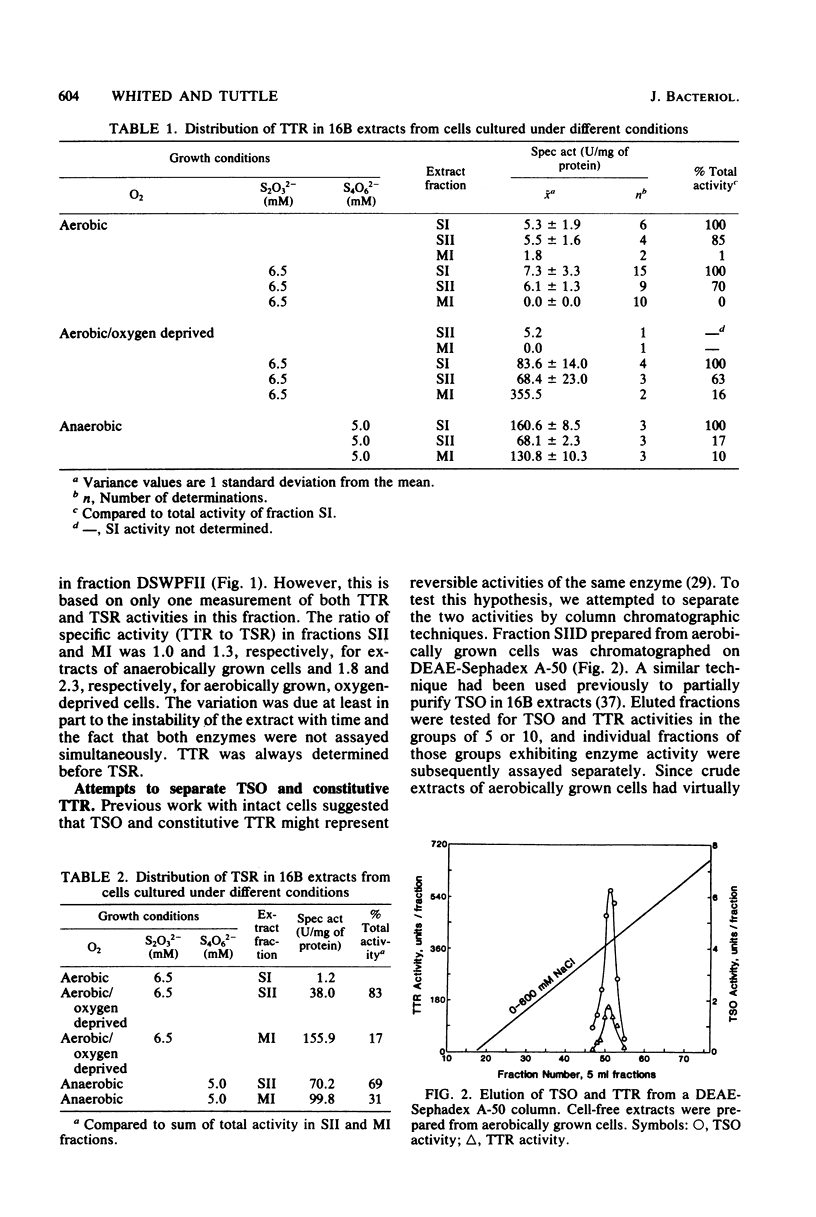
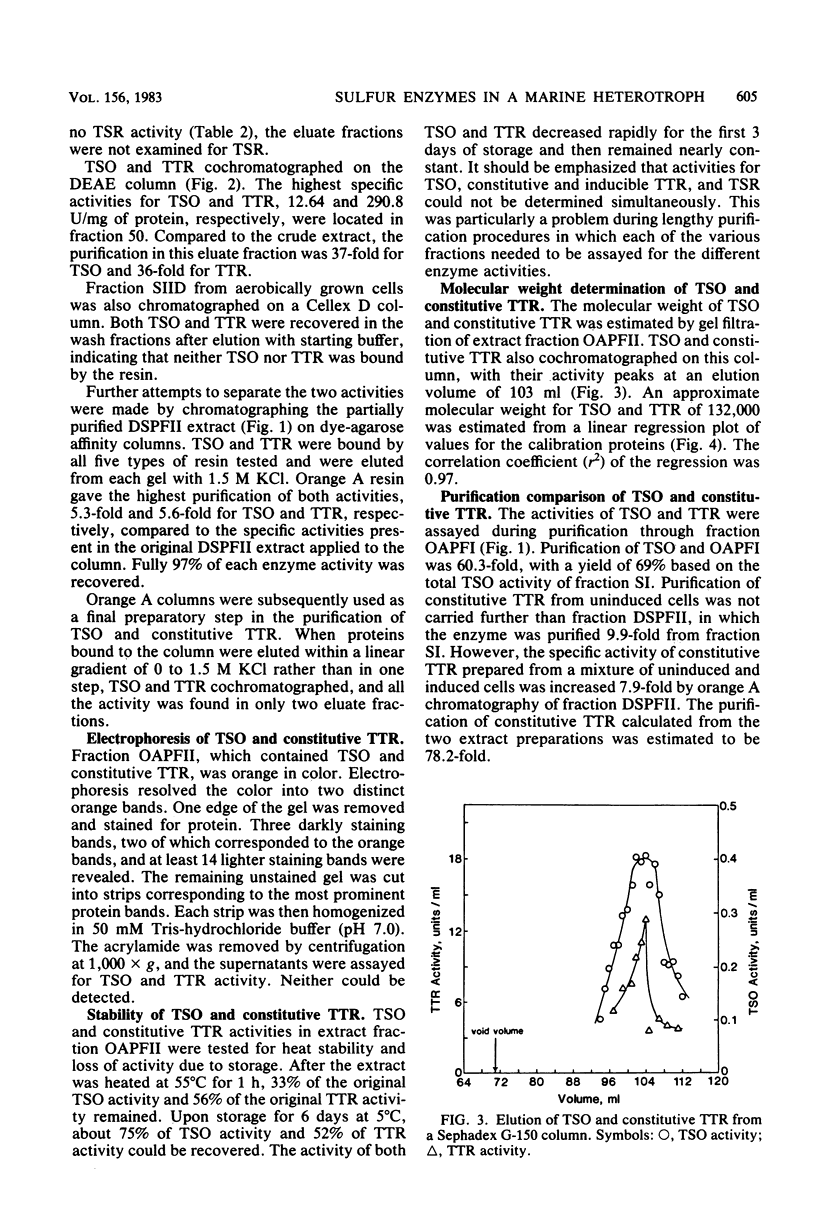
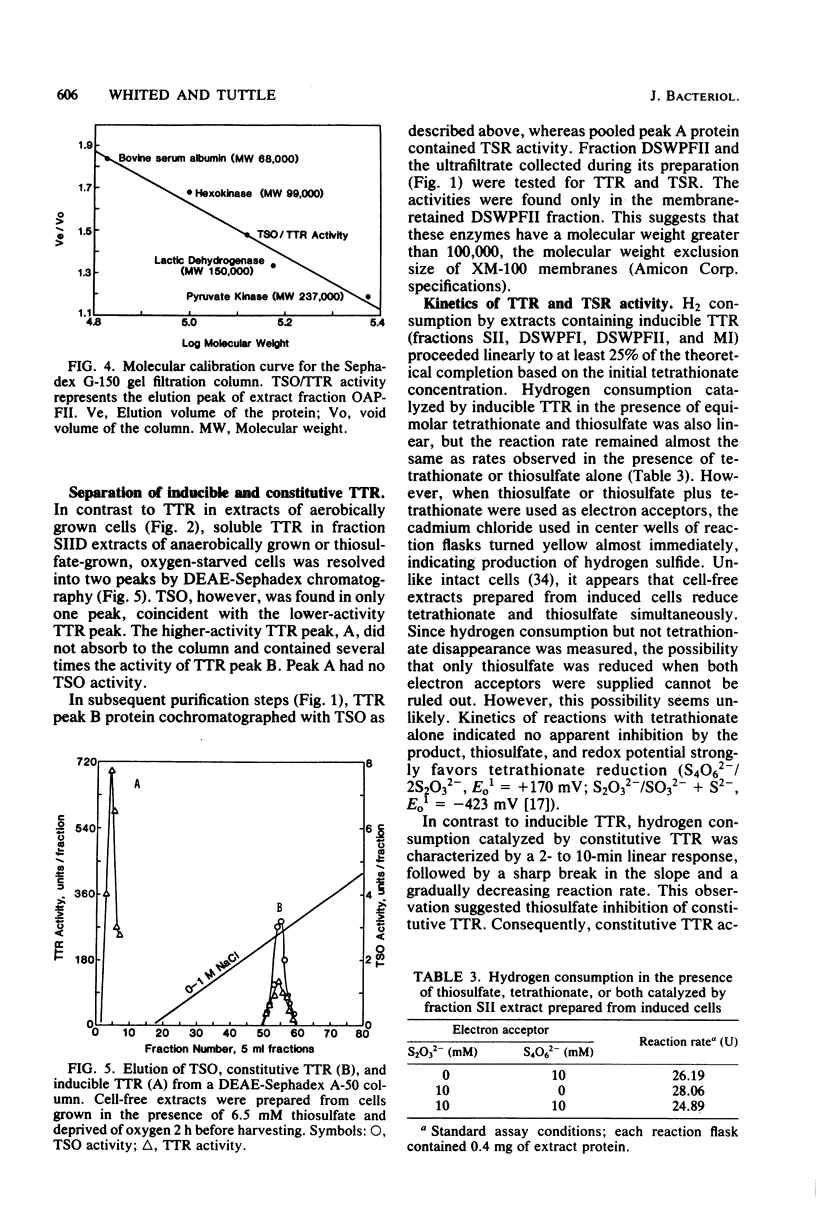
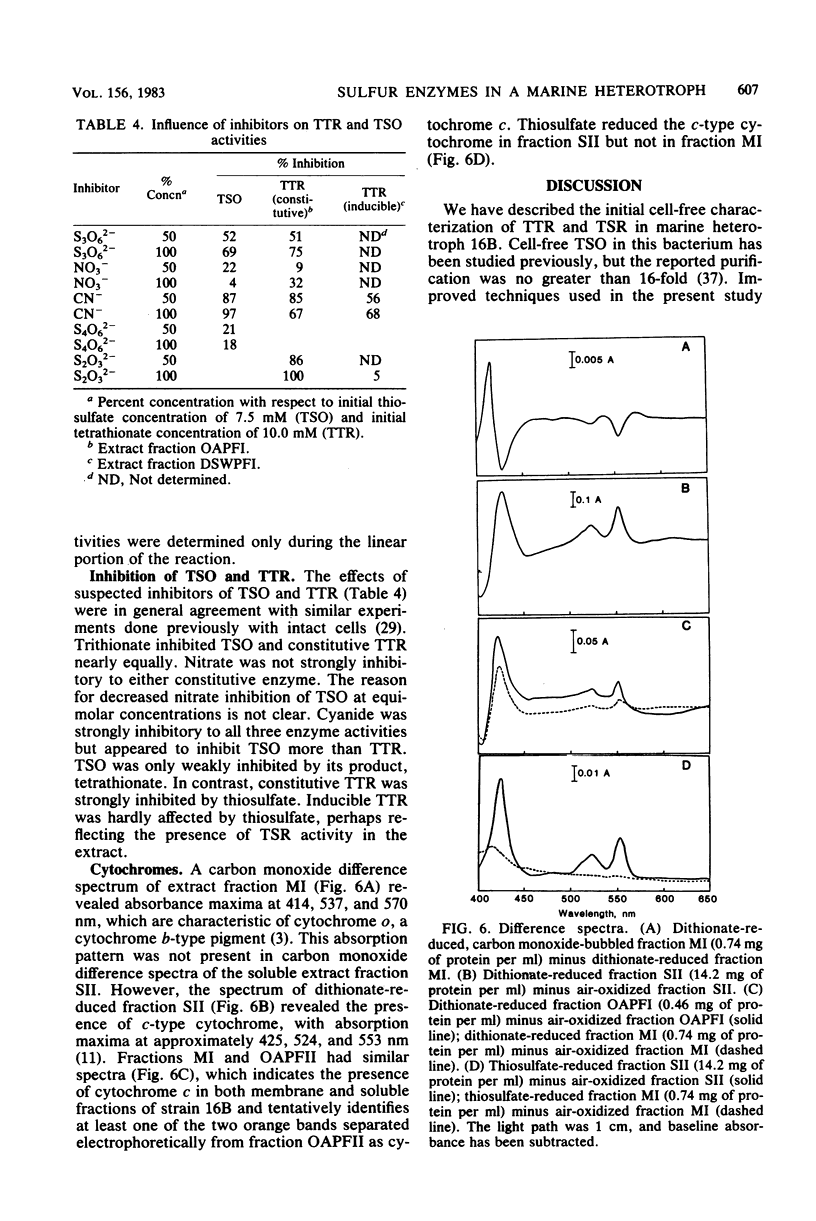
Thiosulfate-oxidizing enzyme (TSO), tetrathionate reductase (TTR), and thiosulfate reductase (TSR) were demonstrated in cell-free extracts of the marine heterotrophic thiosulfate-oxidizing bacterium strain 16B. Extracts prepared from cells cultured aerobically in the absence of thiosulfate or tetrathionate exhibited constitutive TSO and TTR activity which resided in the soluble fraction of ultracentrifuged crude extracts. Constitutive TSO and TTR cochromatographed on DEAE-Sephadex A-50, Cellex D, Sephadex G-150, and orange A dye-ligand affinity gels. Extracts prepared from cells cultured anaerobically with tetrathionate or aerobically with thiosulfate followed by oxygen deprivation showed an 11- to 30-fold increase in TTR activity, with no increase in TSO activity. The inducible TTR resided in both the ultracentrifuge pellet and supernatant fractions and was readily separated from constitutive TSO and TTR in the latter by DEAE-Sephadex chromatography. Inducible TTR exhibited TSR activity, which was also located in both membrane and soluble extract fractions and which cochromatographed with inducible TTR. The results indicate that constitutive TSO and TTR in marine heterotroph 16B represent reverse activities of the same enzyme whose major physiological function is thiosulfate oxidation. Evidence is also presented which suggests a possible association of inducible TTR and TSR in strain 16B.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi J. M., Campbell L. L. STUDIES ON THERMOPHILIC SULFATE-REDUCING BACTERIA III. : Adenosine Triphosphate-sulfurylase of Clostridium nigrificans and Desulfovibrio desulfuricans. J Bacteriol. 1962 Dec;84(6):1194–1201. doi: 10.1128/jb.84.6.1194-1201.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C. Purification and properties of thiosulfate reductase from Desulfovibrio gigas. Arch Microbiol. 1975 Nov 7;105(3):249–256. doi: 10.1007/BF00447143. [DOI] [PubMed] [Google Scholar]
- Kaprálek F., Pichinoty F. The effect of oxygen on tetrathionate reductase activity and biosynthesis. J Gen Microbiol. 1970 Jul;62(1):95–105. doi: 10.1099/00221287-62-1-95. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Darnall D. W. Protein subunits: a table (second edition). Science. 1969 Oct 3;166(3901):126–128. doi: 10.1126/science.166.3901.126. [DOI] [PubMed] [Google Scholar]
- LONDON J., RITTENBERG S. C. PATH OF SULFUR IN SULFIDE AND THIOSULFATE OXIDATION BY THIOBACILLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1183–1190. doi: 10.1073/pnas.52.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. 3. Properties of thiosulfate-oxidizing enzyme and proposed pathway of thiosulfate oxidation. Can J Biochem. 1970 Mar;48(3):355–363. doi: 10.1139/o70-058. [DOI] [PubMed] [Google Scholar]
- Novotný C. Instability of the tetrathionate respiratory chain of Citrobacter freundii. Folia Microbiol (Praha) 1978;23(6):428–432. doi: 10.1007/BF02885570. [DOI] [PubMed] [Google Scholar]
- Ogita Z. I., Markert C. L. A miniaturized system for electrophoresis on polyacrylamide gels. Anal Biochem. 1979 Nov 1;99(2):233–241. doi: 10.1016/s0003-2697(79)80001-9. [DOI] [PubMed] [Google Scholar]
- Oltmann L. F., Schoenmaker G. S., Stouthamer A. H. Solubilization and purification of a cytoplasmic membrane bound enzyme catalyzing tetrathionate and thiosulphate reduction in Proteus mirabilis. Arch Mikrobiol. 1974 Jun 7;98(1):19–30. doi: 10.1007/BF00425264. [DOI] [PubMed] [Google Scholar]
- Oltmann L. F., Stouthamer A. H. Reduction of tetrathionate, trithionate and thiosulphate, and oxidation of sulphide in proteus mirabilis. Arch Microbiol. 1975 Oct 27;105(2):135–142. doi: 10.1007/BF00447128. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., BIGLIARDI-ROUVIER J. [Research on tetrathionate reductase of a facultative anaerobic bacterium]. Biochim Biophys Acta. 1963 Mar 12;67:366–378. doi: 10.1016/0006-3002(63)91843-2. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Wirsen C. O., Jannasch H. W. Chemolithotrophic sulfur-oxidizing bacteria from the galapagos rift hydrothermal vents. Appl Environ Microbiol. 1981 Aug;42(2):317–324. doi: 10.1128/aem.42.2.317-324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. The role of tetrathionate in the oxidation of thiosulphate by Chromatium sp. strain D. J Gen Microbiol. 1966 Mar;42(3):371–380. doi: 10.1099/00221287-42-3-371. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 2. Thiosulphate-oxidizing enzyme. Biochem J. 1961 Apr;78:680–686. doi: 10.1042/bj0780680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A. Metabolism of thiosulfate and tetrathionate by heterotrophic bacteria from soil. J Bacteriol. 1967 Feb;93(2):550–559. doi: 10.1128/jb.93.2.550-559.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H., Holmes P. E., Jannasch H. W. Growth rate stimulation of marine pseudomonads by thiosulfate. Arch Microbiol. 1974;99(1):1–14. doi: 10.1007/BF00696218. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H., Jannasch H. W. Dissimilatory reduction of inorganic sulfur by facultatively anaerobic marine bacteria. J Bacteriol. 1973 Sep;115(3):732–737. doi: 10.1128/jb.115.3.732-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H. Organic carbon utilization by resting cells of thiosulfate-oxidizing marine heterotrophs. Appl Environ Microbiol. 1980 Sep;40(3):516–521. doi: 10.1128/aem.40.3.516-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H., Schwartz J. H., Whited G. M. Some Properties of Thiosulfate-Oxidizing Enzyme from Marine Heterotroph 16B. Appl Environ Microbiol. 1983 Aug;46(2):438–445. doi: 10.1128/aem.46.2.438-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H. Thiosulfate Oxidation and Tetrathionate Reduction by Intact Cells of Marine Pseudomonad Strain 16B. Appl Environ Microbiol. 1980 Jun;39(6):1159–1166. doi: 10.1128/aem.39.6.1159-1166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



